Abstract
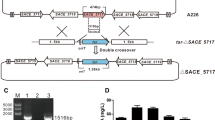
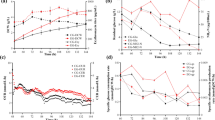
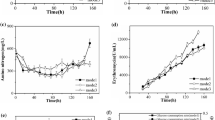
A high erythromycin producing mutant strain Saccharopolyspora erythraea HL3168 E3-ΔmutB was constructed by deleting mutB (SACE_5639) gene encoding the beta subunit of methylmalonyl-CoA mutase of an industrial strain of S. erythraea HL3168 E3. Industrial media and process control strategies were adopted in a 5 L bioreactor for characterizing the physiological parameters. The total erythromycin titer and erythromycin A concentration in mutant were 46.9 (12740.5 μg/mL) and 64.9 % (8094.4 μg/mL) higher than those in original strain, respectively, which were comparable to industrial erythromycin production. The specific glucose and n-propanol consumption rates were increased by 52.4 and 39.8 %, respectively. During the rapid erythromycin synthesis phase, the yield of erythromycin on n-propanol also increased from 24.3 % in control group to 66.9 % in mutant group. Meanwhile, the specific formation rates of methylmalonyl-CoA and propionyl-CoA, two crucial precursors for erythromycin synthesis, were 1.89- and 2.02-folds higher in the mutant strain, respectively.



Similar content being viewed by others
References
Isaac OK, Jos H, Eugene R, Hubert V, Ludo V, Maurice D, Claude P, Pierre P, Georgette L (1985) Antibacterial activities of erythromycins A, B, C, and D and some of their derivatives. Antimicrob Agents Chemother 28:630–633
Chen Y, Deng W, Wu J, Qian J, Chu J, Zhuang Y, Zhang S, Liu W (2008) Genetic modulation of the overexpression of tailoring genes eryK and eryG leading to the improvement of erythromycin A purity and production in Saccharopolyspora erythraea fermentation. Appl Environ Microbiol 74:1820–1828
Zou X, Hang HF, Chu J, Zhuang YP, Zhang SL (2009) Oxygen uptake rate optimization with nitrogen regulation for erythromycin production and scale-up from 50 L to 372 m(3) scale. Bioresour Technol 100:1406–1412
Zhang Q, Chen Y, Hong M, Gao Y, Chu J, Zhuang YP, Zhang SL (2014) The dynamic regulation of nitrogen and phosphorus in the early phase of fermentation improves the erythromycin production by recombinant Saccharopolyspora erythraea strain. Bioresour Bioprocess 1:1–6
Wang Y, Chu J, Zhuang YP, Zhang LX, Zhang SL (2007) Improved production of erythromycin A by expression of a heterologous gene encoding S-adenosylmethionine synthetase. Appl Microbiol Biotechnol 75:837–842
Peter B, Wolfgang M, Pauli TK, James EB (1998) Genetic engineering of an industrial strain of Saccharopolyspora erythraea for stable expression of the Vitreoscilla haemoglobin gene (vhb). Microbiology 144:2441–2448
Jean P, Paul P (1993) Influence of n-propanol on growth and antibiotic production by an industrial strain of Streptomyces erythreus under different nutritional condition. Biotechnol Lett 15:455–460
Konstancja RB, Zbigniew R, Danuta SK, Andrzej R (1973) Limiting reaction in activation of acyl units in biosynthesis of macrolide antibiotic. Am Soc Microbiol 2:162–167
Murli S, Kennedy J, Dayem LC, Carney JR, Kealey JT (2003) Metabolic engineering of Escherichia coli for improved 6-deoxyerythronolide B production. J Ind Microbiol Biotechnol 30:500–509
Chen Y, Huang MZ, Wang ZJ, Chu J, Zhuang YP, Zhang SL (2013) Controlling the feed rate of glucose and propanol for the enhancement of erythromycin production and exploration of propanol metabolism fate by quantitative metabolic flux analysis. Bioprocess Biosyst Eng 36:1445–1453
Reeves AR, Cernota WH, Brikun IA, Wesley RK, Weber JM (2004) Engineering precursor flow for increased erythromycin production in Aeromicrobium erythreum. Metab Eng 6:300–312
Reeves AR, Brikun IA, Cernota WH, Leach BI, Gonzalez MC, Weber JM (2006) Effects of methylmalonyl-CoA mutase gene knockouts on erythromycin production in carbohydrate-based and oil-based fermentations of Saccharopolyspora erythraea. J Ind Microbiol Biotechnol 33:600–609
Weber JM, Cernota WH, Gonzalez MC, Leach BI, Reeves AR, Wesley RK (2012) An erythromycin process improvement using the diethyl methylmalonate responsive (Dmr) phenotype of the Saccharopolyspora erythraea mutB strain. Appl Microbiol Biotechnol 93:1575–1583
Wu JQ, Zhang QL, Deng W, Qian JC, Zhang SL, Liu W (2011) Toward improvement of erythromycin A production in an industrial Saccharopolyspora erythraea strain via facilitation of genetic manipulation with an artificial attB site for specific recombination. Appl Environ Microbiol 77:7508–7516
Bierman M, Logan R, O’Brien K, Seno ET, Rao RN, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49
Hopwood DA, Bibb MJ, Bruton CJ, Kieser T, Chater KF, Smith CP, Kieser HM, Lydiate DJ, Ward JM, Schempf H (2000) Genetic manipulation of streptomyces: a laboratory manual. The John Innes Foundation, Norwich
Matsushima P, Broughton MC, Turner JR, Baltz RH (1994) Conjugal transfer of cosmid DNA from Escherichia coli to Saccharopolyspora spinosa: effects of chromosomal insertions on macrolide A83543 production. Gene 146:39–45
Linde T, Zoglowek M, Lubeck M, Frisvad JC, Lubeck PS (2016) The global regulator LaeA controls production of citric acid and endoglucanases in Aspergillus carbonarius. J Ind Microbiol Biotechnol. doi:10.1007/s10295-016-1781-3
Zou X, Hang HF, Chu J, Zhuang YP, Zhang SL (2009) Enhancement of erythromycin A production with feeding available nitrogen sources in erythromycin biosynthesis phase. Bioresour Technol 100:3358–3365
Chen Y, Wang ZJ, Chu J, Xi BL, Zhuang YP (2015) The glucose RQ-feedback control leading to improved erythromycin production by a recombinant strain Saccharopolyspora erythraea ZL1004 and its scale-up to 372-m(3) fermenter. Bioprocess Biosyst Eng 38:105–112
Chen Y, Wang ZJ, Chu J, Zhuang YP, Zhang SL, Yu XG (2013) Significant decrease of broth viscosity and glucose consumption in erythromycin fermentation by dynamic regulation of ammonium sulfate and phosphate. Bioresour Technol 134:173–179
Liu TT, Wang T, Yang Y, Wang ZJ, Zhuang YP, Chu J, Guo MJ (2016) Low field nuclear magnetic resonance for rapid quantitation of microalgae lipid and its application in high throughput screening. Chin J Biotech. doi:10.13345/j.cjb.150489
Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54:287–301
Baltz RH (2006) Molecular engineering approaches to peptide, polyketide and other antibiotics. Nat Biotechnol 24:1533–1540
Tsuji K, Goetz JF (1978) HPLC as a rapid means of monitoring erythromycin and tetracycline fermentation processes. J Antibiot 31:302–308
Licona-Cassani C, Marcellin E, Quek LE, Jacob S, Nielsen LK (2012) Reconstruction of the Saccharopolyspora erythraea genome-scale model and its use for enhancing erythromycin production. Antonie Van Leeuwenhoek 102:493–502
Zhang G, Yang G, Wang X, Guo Q, Li Y, Li J (2012) Influence of blocking of 2,3-butanediol pathway on glycerol metabolism for 1,3-propanediol production by Klebsiella oxytoca. Appl Biochem Biotechnol 168:116–128
Seo MY, Seo JW, Heo SY, Baek JO, Rairakhwada D, Oh BR, Seo PS, Choi MH, Kim CH (2009) Elimination of by-product formation during production of 1,3-propanediol in Klebsiella pneumoniae by inactivation of glycerol oxidative pathway. Appl Microbiol Biotechnol 84:527–534
Nocon J, Steiger M, Mairinger T, Hohlweg J, Russmayer H, Hann S, Gasser B, Mattanovich D (2016) Increasing pentose phosphate pathway flux enhances recombinant protein production in Pichia pastoris. Appl Microbiol Biotechnol. doi:10.1007/s00253-016-7363-5
Siedler S, Lindner SN, Bringer S, Wendisch VF, Bott M (2013) Reductive whole-cell biotransformation with Corynebacterium glutamicum: improvement of NADPH generation from glucose by a cyclized pentose phosphate pathway using pfkA and gapA deletion mutants. Appl Microbiol Biotechnol 97:143–152
Tao YZ, Liu D, Yan X, Zhou ZH, Lee JK, Yang C (2012) Network identification and flux quantification of glucose metabolism in Rhodobacter sphaeroides under photoheterotrophic H-2-producing conditions. J Bacteriol 194:274–283
Acknowledgments
This work was financially supported by a Grant from the Major State Basic Research Development Program of China (973 Program, No. 2012CB721006) and National Natural Science Foundation of China (No. 21276081).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, C., Hong, M., Chu, J. et al. Blocking the flow of propionate into TCA cycle through a mutB knockout leads to a significant increase of erythromycin production by an industrial strain of Saccharopolyspora erythraea . Bioprocess Biosyst Eng 40, 201–209 (2017). https://doi.org/10.1007/s00449-016-1687-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1687-5