Abstract
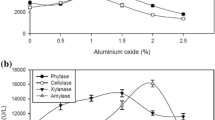
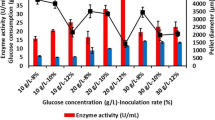
β-mannanase was produced mainly by Aspergillus species and can degrade the β-1,4-mannose linkages of galactomannans. This study was undertaken to enhance mannanase production using talcum and aluminum oxide as the microparticles, which control cell morphology of recombinant Aspergillus sojae in glucose and carob extract medium. Both microparticles improved fungal growth in glucose and carob pod extract medium. Aluminum oxide (1 g/L) was the best agent for glucose medium which resulted in 514.0 U/ml. However, the highest mannanase activity was found as 568.7 U/ml with 5 g/L of talcum in carob extract medium. Increase in microparticle concentration resulted in decreasing the pellet size diameter. Furthermore, more than 10 g/L of talcum addition changed the filamentous fungi growth type from pellet to pellet/mycelium mixture. Results showed that right type and concentration of microparticle in fermentation media improved the mannanase activity and production rate by controlling the growth morphology.






Similar content being viewed by others
References
Aehle W (2004) Enzymes in industry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Rosengren A, Reddy SK, Sjöberg JS, Aurelius O, Logan DT, Kolenova K, Stalbrand H (2014) An Aspergillus nidulans β-mannanase with high transglycosylation capacity revealed through comparative studies within glycosidase family 5. Appl Microbiol Biot 98(24):10091–10104
Van Zyl WH, Rose SH, Trollope K, Görgens JF (2010) Fungal β-mannanases: mannan hydrolysis, heterologous production and biotechnological applications. Process Biochem 45:1203–1213
Lu H, Luo H, Shi P, Huang H, Meng K, Yang P, Yao B (2014) A novel thermophilic endo-β-1,4 mannanase from Aspergillus nidulans XZ3: functional roles of carbohydrate-binding module and Thr/Ser rich linker region. Appl Microbiol Biot 98:2155–2163
Lin SS, Dou WF, Xu HY, Li HZ, Xu ZH, Ma YH (2007) Optimization of medium composition for the production of alkaline β-mannanase by alkaliphilic Bacillus sp. N16-5 using response surface methodology. Appl Microbiol Biot 75(5):1015–1022
Huang JL, Bao LX, Zou HY, Che SG, Wang GX (2012) High-level production of a cold-active β-mannanase from Bacillus subtilis Bs5 and its molecular cloning and expression. Mol Genet Microbiol Virol 27(4):147–153
Feng Y, He Z, Ong SL, Hu J, Zhang Z, Ng WJ (2003) Optimization of agitation, aeration, and temperature conditions for maximum β-mannanase production. Enzyme Microb Tech 32:282–289
Katrolia P, Yan Q, Zhang P, Zhou P, Yang S, Jiang Z (2013) Gene cloning and enzymatic characterization of an alkali-tolerant endo-1,4-β-mannanase from Rhizomucor miehei. J Agr Food Chem 61:394–401
Yin JS, Liang QL, Li DM, Sun ZT (2012) Optimization of production conditions for β-mannanase using apple pomace as raw material in solid-state fermentation. Ann Microbiol 63:101–108
Wang J, Shao Z, Hong Y, Li C, Fu X, Liu Z (2010) A novel β-mannanase from Pantoea agglomerans A021: gene cloning, expression, purification and characterization. World J Microbiol Biotechnol 26:1777–1784
Chen X, Cao Y, Ding Y, Lu W, Li D (2007) Cloning, functional expression and characterization of Aspergillus sulphures β-mannanase in Pichia pastoris. J Biotechnol 128:452–461
Luo H, Wang K, Huang H, Shi P, Yang P, Yao B (2012) Gene cloning, expression, and biochemical characterization of an alkali-tolerant β-mannanase from Humicola insolens Y1. J Ind Microbiol Biot 39:547–555
Wang Y, Shi P, Luo H, Bai Y, Huang H, Yang P, Xiong H, Yao B (2012) Cloning, over-expression and characterization of an alkali-tolerant endo-β-1,4-mannanase from Penicillium freii F63. J Biosci Bioeng 113(6):710–714
Großwindhager C, Sachslehner A, Nidetzky B, Haltrich D (1999) Endo-β-1,4-D-mannanase is efficiently produced by Sclerotium (Athelia) rolfsii under derepressed conditions. J Biotechnol 67(2–3):189–203
Ozturk B, Cekmecelioglu D, Ogel ZB (2010) Optimal conditions for enhanced β-mannanase production by recombinant Aspergillus sojae. J Mol Catal B Enzym 64:135–139
Turhan I, Bialka KL, Demirci A, Karhan M (2010) Ethanol production from carob extract by using Saccharomyces cerevisiae. Bioresour Tech 101:5290–5296
Yatmaz E, Turhan I, Karhan M (2013) Optimization of ethanol production from carob pod extract using immobilized saccharomyces cerevisiae cells in a stirred tank bioreactor. Bioresour Tech 135:365–371
Kaup BA, Ehrich K, Pescheck M, Schrader J (2008) Microparticle-enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng 99(3):491–498
Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittman C (2012) Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng 105(6):1058–1068
Driouch H, Sommer B, Wittman C (2010) Morphology engineering of Aspergillus niger for improved enzyme production. Biotechnol Bioeng 109(2):462–471
Coban HB, Demirci A, Turhan I (2015) Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng 38(6):1075–1080
Coban HB, Demirci A, Turhan I (2015) Enhanced Aspergillus ficuum phytase production in fed-batch and continuous fermentations in the presence of talcum microparticles. Bioprocess Biosyst Eng 38(8):1431–1436
Coban HB, Demirci A (2016) Enhancement and modeling of microparticle-added Rhizopus oryzae lactic acid production. Bioprocess Biosyst Eng 39:323–330
Duruksu G, Ozturk B, Biely P, Bakır U, Ogel ZB (2009) Cloning, expression and characterization of endo-beta-1,4-mannanase from Aspergillus fumigatus in Aspergillus sojae and Pichia pastoris. Biotechnol Progr 25:271–276
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing methods for assay of xylanase activity. J Biotechnol 23:257–270
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426
Acknowledgments
This study was supported by the TUBITAK foundation (Project number: 112 O 167) and Akdeniz University Research Foundation (Project numbers: 2013.12.0102.017 and FDK-2015-681).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yatmaz, E., Karahalil, E., Germec, M. et al. Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst Eng 39, 1391–1399 (2016). https://doi.org/10.1007/s00449-016-1615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1615-8