Abstract
Based on the screening of biocatalysts and reaction conditions including solvent, water content, temperature, enzyme loading, and reaction time, lipase from porcine pancreas (PPL) showed the prominent promiscuity for the Knoevenagel condensation between 1,3-dihydroindol-2-one heterocycle and aromatic aldehydes. Under the optimized procedure, both electron-withdrawing and electron-donating substituent of aldehydes substrates could react efficiently, and benzylidene-indolin-2-ones were obtained in excellent yields (75.0–96.6 %).
Graphical abstract
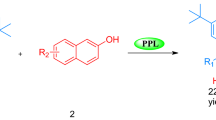
Benzylidene-indolin-2-ones derivatives were efficiently synthesized by the Knoevenagel condensation between various aromatic aldehydes and 1,3-dihydroindol-2-one catalyzed by lipase from porcine pancreas with excellent yields obtained.





Similar content being viewed by others
References
Mukherjee R, Jaggi M, Rajendran P, Siddiqui MJA, Srivastava SK, Vardhan A, Burman AC (2004) Betulinic acid and its derivatives as anti- angiogenic agent. Bioorg Med Chem Lett 14:2181–2184
Xu TT, Wang SY, Ji SJ (2006) Facile synthesis of Z/E-3-arylmethylidene-2,3-dihydro- indol-2-one under solvent-free conditions. Chin J Org Chem 26:1414–1417
Porcs-Makkay M, Volk B, Kapiller-Dezsofi R, Mezei T, Simig G (2004) New routes to oxindole derivatives. Monatsh Chem 135:697–711
Du TP, Zhu GG, Zhou J (2012) A facile method for the synthesis of 3-alkyloxindole. Lett Org Chem 9:225–232
Botta G, De Santis LP, Saladino R (2012) Current advances in the synthesis and antitumoral activity of SIRT1-2 inhibitors by modulation of p53 and pro-apoptotic proteins. Curr Med Chem 19:5871–5884
Zhang W, Go ML (2009) Functionalized 3-benzylidene-indolin-2-ones: inducers of NAD(P)H-quinone oxidoreductase 1 (NQO1) with antiproliferative activity. Bioorg Med Chem 17:2077–2090
Olgen S, Gotz C, Jose J (2007) Synthesis and biological evaluation of 3-(substituted-benzylidene)-1,3-dihydro-indolin derivatives as human protein kinase CK2 and p60(c-Src) tyrosine kinase inhibitors. Biol Pharm Bull 30:715–718
Kuchar M, Steinbach J, Wuest F (2009) Synthesis and radiopharmacological investigation of 3-[4’-[F-18] fluorobenzylidene] indolin-2-one as possible tyrosine kinase inhibitor. Bioorg Med Chem 17:7732–7742
Ding K, Wang GP, Deschamps JR, Parrish DA, Wang SM (2005) Synthesis of spirooxindoles via asymmetric 1,3-dipolar cycloaddition. Tetrahedron Lett 46:5949–5951
Villemin D, Martin B (1998) Potassium fluoride on alumina: dry synthesis of 3-arylidene-1,3-dihydro-indol-2-one under microwave irradiation. Synth Commun 28:3201–3208
Wang XS, Zeng ZS, Li YL, Shi DQ, Tu SJ, Wei XY, Zong ZM (2005) Synthesis of E-arylidene-2,3-dihydroindol-2-one derivatives in aqueous media catalyzed by triethylbenzylammonium chloride phase-transfer catalyst. Chin J Org Chem 25:1598–1601
Hu Y, Kang H, Zeng BW, Wei P, Huang H (2008) Facile synthesis of 3-arylidene-1,3-dihydroindol-2-ones catalysed by a Bronsted acidic ionic liquid. J Chem Res 11:642–643
Friedrich S, Hahn F (2015) Opportunities for enzyme catalysis in natural product chemistry. Tetrahedron 71:1473–1508
Reetz MT (2013) Biocatalysis in organic chemistry and biotechnology: past, present, and future. J Am Chem Soc 135:12480–12496
Miao YF, Rahimi M, Geertsema EM, Poelarends GJ (2015) Recent developments in enzyme promiscuity for carbon-carbon bond-forming reactions. Curr Opin Chem Biol 25:115–123
Kapoor M, Gupta MN (2012) Lipase promiscuity and its biochemical applications. Process Biochem 47:555–569
Ding Y, Huang H, Hu Y (2013) New progress on lipases catalyzed C–C bond formation reactions. Chin J Org Chem 33:905–914
Ding Y, Ni X, Gu MJ, Li S, Huang H, Hu Y (2015) Knoevenagel condensation of aromatic aldehydes with active methylene compounds catalyzed by lipoprotein lipase. Catal Commun 64:101–104
Wang Z, Wang CY, Wang HR, Zhang H, Sun YL, Ji TF, Wang L (2014) Lipase-catalyzed Knoevenagel condensation between α, β-unsaturated aldehydes and active methylene compounds. Chin Chem Lett 25:802–804
Jiang L, Yu HW (2014) Enzymatic promiscuity: Escherichia coli BioH esterase-catalysed Aldol reaction and Knoevenagel reaction. Chem Res Chin Univ 30:289–292
Lai YF, Zheng H, Chai SJ, Zhang PF, Chen XZ (2010) Lipase-catalysed tandem Knoevenagel condensation and esterification with alcohol cosolvents. Green Chem 12:1917–1918
Hu Y, Jiang XJ, Wu SW, Jiang L, Huang H (2013) Synthesis of vitamin E succinate by interfacial activated Candida rugosa lipase encapsulated in sol–gel materials. Chin J Catal 34:1608–1616
Liu WM, Hu Y, Jiang L, Zou B, Huang H (2012) Synthesis of methyl (R)-3-(4-fluorophenyl)glutarate via enzymatic desymmetrization of a prochiral diester. Process Biochem 47:1037–1041
Jiang XJ, Hu Y, Jiang L, Zou B, Huang H (2013) Optimization of enzymatic synthesis of l-ascorbyl palmitate by solvent engineering and statistical experimental designs. Biotechnol Bioproc Eng 18:350–357
Laane C, Boeren S, Vos K, Veeger C (2009) Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 102:2–8
Salihu A, Alam MZ (2015) Solvent tolerant lipases: a review. Process Biochem 50:86–96
Doukyu N, Ogino H (2010) Organic solvent-tolerant enzymes. Biochem Eng J 48:270–282
Hu W, Guan Z, Deng X, He YH (2012) Enzyme catalytic promiscuity: the papain-catalyzed Knoevenagel reaction. Biochimie 94:656–661
Zaks A, Klibanov AM (1988) The effect of water on enzyme action in organic media. J Biol Chem 263:8017–8021
Narayan VS, Klibanov AM (1993) Are water-immiscibility and apolarity of the solvent relevant to enzyme efficiency? Biotechnol Bioeng 41:390–393
Li K, He T, Li C, Feng XW, Wang N, Yu XQ (2009) Lipase-catalysed direct Mannich reaction in water: utilization of biocatalytic promiscuity for C–C bond formation in a “one-pot” synthesis. Green Chem 11:777–779
Li C, Feng XW, Wang N, Zhou YJ, Yu XQ (2008) Biocatalytic promiscuity: the first lipase-catalysed asymmetric aldol reaction. Green Chem 10:616–618
Sun FL, Xu G, Wu JP, Yang LR (2006) Efficient lipase-catalyzed kinetic resolution of 4-arylmethoxy-3-hydroxybutanenitriles: application to an expedient synthesis of a statin intermediate. Tetrahedron Asymmetry 17:2907–2913
Acknowledgments
This research was financially supported by the Hi-Tech Research and Development Program of China (Grant No. 2011AA02A209) and National Science Foundation for Distinguished Young Scholars of China (Grant No. 21225626).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ding, Y., Xiang, X., Gu, M. et al. Efficient lipase-catalyzed Knoevenagel condensation: utilization of biocatalytic promiscuity for synthesis of benzylidene-indolin-2-ones. Bioprocess Biosyst Eng 39, 125–131 (2016). https://doi.org/10.1007/s00449-015-1496-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1496-2