Abstract
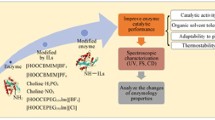
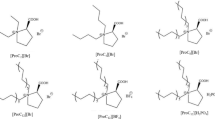
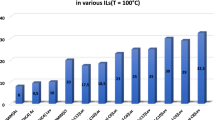
Chemical modification of lysine residues in Candida rugosa lipase (CRL) was carried out using five different functional ionic liquids, and about 15.4–25.0 % of the primary amino groups of lysine were modified. Enzymatic properties of the native and modified CRLs were investigated in olive oil hydrolysis reaction. Improved thermal stability, catalytic activity in organic solvents, and adaptability to temperature and pH changes were achieved compared with the native enzyme. CRL modified by [choline][H2PO4] showed the best results, bearing a maximum improvement of 16.7 % in terms of relative activity, 5.2-fold increase in thermostability (after incubation at 45 °C for 5 h), and 2.3-fold increase in activity in strong polar organic solvent (80 % dimethyl sulfoxide) compared with the native enzyme. The results of ultraviolet, circular dichroism and fluorescence spectroscopy suggested that the change of the secondary and tertiary structures of CRL caused by the chemical modification resulted in the enhancement of enzymatic performance. The modification of CRL with functional ionic liquids was proved to be a novel and efficient method for improving the enzymatic properties of CRL.










Similar content being viewed by others
References
Seelig B, Szostak JW (2007) Selection and evolution of enzymes from a partially randomized non-catalytic scaffold. Nature 448:828–831
Zheng GW, Xu JH (2011) New opportunities for biocatalysis: driving the synthesis of chiral chemicals. Curr Opin Biotechnol 22:784–792
Goswami D, Basu JK, De S (2013) Lipase applications in oil hydrolysis with a case study on castor oil: a review. Crit Rev Biotechnol 33:1–16
Ding Y, Huang H, Hu Y (2013) New progress on lipases catalyzed C–C bond formation reactions. Chin J Org Chem 33:905–914
Deive FJ, Álvarez MS, Sanromán MA, Longo MA (2013) North Western Spain hot springs are a source of lipolytic enzyme-producing thermophilic microorganisms. Bioprocess Biosyst Eng 36:239–250
Hult K, Maurer S, Hamberg A (2012) Rational engineering of Candida antarctica lipase B for selective monoacylation of diols. Chem Commun 48:10013–10015
Durand E, Lecomte J, Barea B, Piombo G, Dubreucq E, Villeneuve P (2012) Evaluation of deep eutectic solvents as new media for Candida antarctica B catalyzed reactions. Process Biochem 47:2081–2089
Forsyth C, Patwardhan SV (2013) Controlling performance of lipase immobilised on bioinspired silica. J Mater Chem 1:1164–1174
Díaz-Rodríguez A, Davis BG (2011) Chemical modification in the creation of novel biocatalysts. Curr Opin Chem Biol 15:211–219
Cowan DA, Fernandez-Lafuente R (2011) Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb Technol 49:326–346
Chalker JM, Bernardes GJ, Lin YA, Davis BG (2009) Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem Asian J 4:630–640
Han D, Tang B, Lee YR, Row KH (2012) Application of ionic liquid in liquid phase microextraction technology. J Sep Sci 35:2949–2961
Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37:123–150
Zou B, Hu Y, Yu DH, Jiang L, Liu WM, Song P (2011) Functionalized ionic liquid modified mesoporous silica SBA-15: a novel, designable and efficient carrier for porcine pancreas lipase. Colloids Surf B 88:93–99
Zou B, Hu Y, Yu DH, Xia JJ, Tang SS, Liu WM, Huang H (2010) Immobilization of porcine pancreatic lipase onto ionic liquid modified mesoporous silica SBA-15. Biochem Eng J 53:150–153
Hu Y, Tang SS, Jiang L, Zou B, Yang J, Huang H (2012) Immobilization of Burkholderia cepacia lipase on functionalized ionic liquids modified mesoporous silica SBA-15. Process Biochem 47:2291–2299
Yang J, Hu Y, Jiang L, Zou B, Jia R, Huang H (2013) Enhancing the catalytic properties of porcine pancreatic lipase by immobilization on SBA-15 modified by functionalized ionic liquid. Biochem Eng J 70:46–54
Jia R, Hu Y, Liu L, Jiang L, Zou B, Huang H (2013) Enhancing catalytic performance of porcine pancreatic lipase by covalent modification using functional ionic liquids. ACS Catal 3:1976–1983
Smith PK, Frohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Habeeb AF (1966) Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem 14:328–336
Monier M, Wei Y, Sarhan A (2010) Evaluation of the potential of polymeric carriers based on photo-crosslinkable chitosan in the formulation of lipase from Candida rugosa immobilization. J Mol Catal B Enzym 63:93–101
Zhao H (2006) Are ionic liquids kosmotropic or chaotropic? An evaluation of available thermodynamic parameters for quantifying the ion kosmotropicity of ionic liquids. J Chem Technol Biotechnol 81:877–891
Zhao H, Olubajo O, Song Z, Sims AL, Person TE, Lawal RA, Holley LA (2006) Effect of kosmotropicity of ionic liquids on the enzyme stability in aqueous solutions. Bioorg Chem 34:15–25
Yang Z (2009) Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis. J Biotechnol 144:12–22
Zhao H (2010) Methods for stabilizing and activating enzymes in ionic liquids—a review. J Chem Technol Biotechnol 85:891–907
Hernáiz MJ, Sánchez-Montero JM, Sinisterra JV (1999) Modification of purified lipases from Candida rugosa with polyethylene glycol: a systematic study. Enzyme Microb Technol 24:181–190
de la Casa RM, Guisán JM, Sánchez-Montero JM, Sinisterra JV (2002) Modification of the activities of two different lipases from Candida rugosa with dextrans. Enzyme Microb Technol 30:30–40
Park K, Kim H, Maken S, Kim Y, Min B, Park J (2005) Characteristics of the lipase from Candida rugosa modified with copolymers of polyoxyethylene derivative and maleic acid anhydride. Korean J Chem Eng 22:412–417
Bian W, Lou LL, Yan B, Zhang C, Wu S, Liu S (2011) Immobilization of papain by carboxyl-modified SBA-15: rechecking the carboxyl after excluding the contribution of H2SO4 treatment. Micropor Mesopor Mater 143:341–347
Liu JZ, Wang TL, Huang MT, Song HY, Weng LP, Ji LN (2006) Increased thermal and organic solvent tolerance of modified horseradish peroxidase. Protein Eng Des Sel 19:169–173
Szabó A, Kotormán M, Laczkó I, Simon LM (2009) Improved stability and catalytic activity of chemically modified papain in aqueous organic solvents. Process Biochem 44:199–204
Xiong Y, Gao J, Zheng J, Deng N (2011) Effects of succinic anhydride modification on laccase stability and phenolics removal efficiency. Chin J Catal 32:1584–1591
Freitas DD, Abrahão-Neto J (2010) Biochemical and biophysical characterization of lysozyme modified by PEGylation. Int J Pharm 392:111–117
Liu JZ, Wang M (2007) Improvement of activity and stability of chloroperoxidase by chemical modification. BMC Biotechnol 7:23–30
Nordwald EM, Kaar JL (2013) Stabilization of enzymes in ionic liquids via modification of enzyme charge. Biotechnol Bioeng 110:2352–2360
Acknowledgments
This research was supported by the National Science Foundation for Distinguished Young Scholars of China (No. 21225626), the National Natural Science Foundation of China for Young Scholars (Grant No. 20906049), the National Basic Research Program of China (Grant No. 2011CB710800), the Hi-Tech Research and Development Program of China (863 Program, 2011AA02A209).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Y., Yang, J., Jia, R. et al. Chemical modification with functionalized ionic liquids: a novel method to improve the enzymatic properties of Candida rugosa lipase. Bioprocess Biosyst Eng 37, 1617–1626 (2014). https://doi.org/10.1007/s00449-014-1134-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1134-4