Abstract
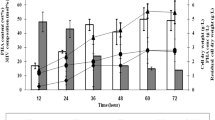
The biotransformation of levulinic acid to 4-valerolactone (4VL) is pH-dependent and equilibrium limited, distinct from the more common irreversible biotransformations that are constrained by product toxicity or biocatalyst inhibition. Our processing strategy for this system was to selectively remove the product, 4VL, which is in equilibrium with its precursor, 4-hydroxyvalerate (4HV), to pull the reaction to a greater extent of conversion. 4VL is challenging to separate from the aqueous phase due to its water miscibility, necessitating the use of water-absorbing polymers to provide affinity toward the hydrophilic product. Manipulating the composition of copolymers, thereby varying the architecture of polymer chains, conferred drastically different extents of water absorption and caused different biotransformation outcomes. A custom-synthesized random copolymer designed to maximize the proportion of material with affinity for the solute had high water uptake, which resulted in the poor selectivity for the target molecule relative to its precursor. Conversely, a moderately water-absorbing commercial segmented block copolymer, Hytrel® 8206, demonstrated selectivity toward 4VL relative to its precursor, 4HV, and increased 4VL production by approximately 30 % by shifting the equilibrium toward the product. This work has shown that water absorption is an important, previously neglected criterion in evaluating polymer affinity and selectivity toward hydrophilic target molecules.




Similar content being viewed by others
References
Verevkin SP, Emel’yanenko VN (2012) Renewable platform-chemicals and materials: thermochemical study of levulinic acid. J Chem Thermodyn 46:94–98
Chalid M, Heeres H, Broekhuis A (2012) Green polymer precursors from biomass-based levulinic acid. Procedia Chem 4:260–267
Horváth IT, Mehdi H, Fábos V, Boda L, Mika LT (2007) γ-Valerolactone—a sustainable liquid for energy and carbon-based chemicals. Green Chem 10:238–242
Straathof AJJ (2003) Auxiliary phase guidelines for microbial biotransformations of toxic substrate into toxic product. Biotechnol Prog 19:755–762
Götz K, Liese A, Ansorge-Schumacher M, Hilterhaus L (2013) A chemo-enzymatic route to synthesize (S)-γ-valerolactone from levulinic acid. Appl Microbiol Biotechnol 97(9):3865–3873
Martin CH, Wu D, Prather KLJ (2010) Integrated bioprocessing for the pH-dependent production of 4-valerolactone from levulinate in Pseudomonas putida KT2440. Appl Environ Microbiol 76:417
Teiber JF, Draganov DI, Du BNL (2003) Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3. Biochem Pharmacol 66:887–896
Efe C, Straathof AJJ, van der Wielen LAM (2007) Options for biochemical production of 4-hydroxybutyrate and its lactone as a substitute for petrochemical production. Biotechnol Bioeng 99:1392–1406
Malinowski JJ (2001) Two-phase partitioning bioreactors in fermentation technology. Biotechnol Adv 19:525–538
Li A, Zhang Q, Chen J, Fei Z, Long C, Li W (2001) Adsorption of phenolic compounds on Amberlite XAD-4 and its acetylated derivative MX-4. React Funct Polym 49:225–233
Phillips T, Chase M, Wagner S, Renzi C, Powell M, DeAngelo J, Michels P (2013) Use of in situ solid-phase adsorption in microbial natural product fermentation development. J Ind Microbiol Biotechnol 40:1–15
Nielsen DR, Prather KJ (2009) In situ product recovery of n-butanol using polymeric resins. Biotechnol Bioeng 102:811–821
Wang Z, Dai Z (2010) Extractive microbial fermentation in cloud point system. Enzyme Microb Technol 46:407–418
Craig T, Daugulis AJ (2013) Polymer characterization and optimization of conditions for the enhanced bioproduction of benzaldehyde by Pichia pastoris in a two-phase partitioning bioreactor. Biotech Bioeng 110:1098–1105
Hosaka S, Ozawa H, Tanzawa H (1979) Controlled release of drugs from hydrogel matrices. J Appl Polym Sci 23:2089–2098
Prpich GP, Daugulis AJ (2007) A novel solid–liquid two-phase partitioning bioreactor for the enhanced bioproduction of 3-methylcatechol. Biotechnol Bioeng 98:1008–1016
Morrish JLE, Daugulis AJ (2008) Improved reactor performance and operability in the biotransformation of carveol to carvone using a solid–liquid two-phase partitioning bioreactor. Biotechnol Bioeng 101:946–956
Khan TR, Daugulis AJ (2010) Application of solid–liquid TPPBs to the production of l-phenylacetylcarbinol from benzaldehyde using Candida utilis. Biotechnol Bioeng 107:633
Parent JS, Capela M, Dafoe JT, Daugulis AJ (2012) A first principles approach to identifying polymers for use in two-phase partitioning bioreactors. J Chem Technol Biot 87:1059–1065
Various (1999) Polymer Handbook. In: Brandrup J, Immergut EH, Grulke EA (eds) Solubility parameter values, 4th edn. Wiley, Hoboken
Amsden B, Lau A (2008) Siloxane-based copolymers for use in two-phase partitioning bioreactors. Can J Chem Eng 86:1–5
Ju H, McCloskey BD, Sagle AC, Wu YH, Kusuma VA, Freeman BD (2008) Crosslinked poly (ethylene oxide) fouling resistant coating materials for oil/water separation. J Membr Sci 307:260–267
Ping Z, Nguyen Q, Chen S, Zhou J, Ding Y (2001) States of water in different hydrophilic polymers—DSC and FTIR studies. Polymer 42:8461–8467
Tufvesson P, Lima-Ramos J, Jensen JS, Al-Haque N, Neto W, Woodley JM (2011) Process considerations for the asymmetric synthesis of chiral amines using transaminases. Biotechnol Bioeng 108:1479–1493
Ramström O, Ye L, Krook M, Mosbach K (1998) Applications of molecularly imprinted materials as selective adsorbents: emphasis on enzymatic equilibrium shifting and library screening. Chromatographia 47(7–8):465–469
Valentin HE, Schönebaum A, Steinbüchel A (1992) Identification of 4-hydroxyvaleric acid as a constituent of biosynthetic polyhydroxyalkanoic acids from bacteria. Appl Microbiol Biotechnol 36:507–514
Malinowski JJ, Daugulis AJ (1994) Salt effects in extraction of ethanol, 1-butanol and acetone from aqueous solutions. AIChE J 40:1459–1465
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Hu Q, Meng Y, Sun T, Mahmood Q, Wu D, Zhu J, Lu G (2011) Kinetics and equilibrium adsorption studies of dimethylamine (DMA) onto ion-exchange resin. J Hazard Mater 185:677–681
Nielsen DR, Prather KJ (2008) In situ product recovery of n-butanol using polymeric resins. Biotechnol Bioeng 102:811–821
Hytrel thermoplastic elastomer design guide—Module V. http://www2.dupont.com/Plastics/en_US/assets/downloads/design/H81098.pdf. Accessed 13 April 2013
Rehmann L, Sun B, Daugulis AJ (2007) Polymer selection for biphenyl degradation in a solid-liquid two-phase partitioning bioreactor. Biotechnol Prog 23:814–819
Gao F, Daugulis AJ (2010) Polymer-solute interactions in solid-liquid two-phase partitioning bioreactors. J Chem Technol Biot 85:302–306
Rehmann L, Prpich GP, Daugulis AJ (2008) Remediation of PAH contaminated soils: application of a solid-liquid two-phase partitioning bioreactor. Chemosphere 73:798–804
Acknowledgments
The authors gratefully acknowledge Dr. Kristala Prather of Massachusetts Institute of Technology for providing the engineered biocatalyst, and Dr. Collin Martin (MIT) for his helpful discourse. The authors also thank Dr. Shailesh Doshi of DuPont Canada for providing polymers and for his stimulating discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dafoe, J.T., Daugulis, A.J. Production of 4-valerolactone by an equilibrium-limited transformation in a partitioning bioreactor: impact of absorptive polymer properties. Bioprocess Biosyst Eng 37, 533–542 (2014). https://doi.org/10.1007/s00449-013-1020-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1020-5