Abstract
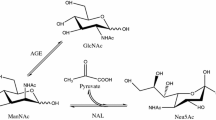
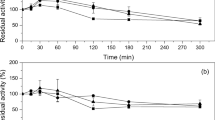
To improve the properties of the immobilised 2-deoxy-d-ribose-5-phosphate aldolase (DERA), unreacted functional groups on support surface were blocked with amino acids. The relative activities of the immobilised enzyme were 144.7 and 141.9% when the post-immobilisation modification was done with Arg and Phe, respectively. The residual activity of immobilised DERA after heating at 60 °C for 120 min was 65.1% when Phe and Val were used as the blocking amino acids, a 2.0- and 2.87-fold increase over that of the immobilised (no post-immobilisation blocking) and free DERA. Immobilised DERA maintained maximal activity in 2-deoxyribose-5-phosphate (DR5P) synthesis up to 600 mM of acetaldehyde, which was much higher than the amount of acetaldehyde tolerated by free enzyme (300 mM). This superior resistance to high acetaldehyde concentrations would accelerate the DR5P reaction by shifting the reaction equilibrium towards the product. The results from this study suggest that the novel immobilised DERA may be useful for industrial applications.






Similar content being viewed by others
References
Dean SM, Greenberg WA, Wong CH (2007) Recent advances in aldolase-catalyzed asymmetric synthesis. Adv Synth Cata 349:1308–1320
Greenberg WA, Varvak A, Hanson SR, Wong K, Huang H, Chen P, Burk MJ (2004) Development of an efficient, scalable, aldolase-catalyzed process for enantioselective synthesis of statin intermediates. Proc Natl Acad Sci USA 101:5788–5793
Chen LR, Dumas DP, Wong CH (1992) Deoxyribose 5-phosphate aldolase as a catalyst in asymmetric aldol condensation. J Am Chem Soc 114:741–748
Patel JM (2009) Biocatalytic synthesis of atorvastatin intermediates. J Mol Catal B: Enzym 61:123–128
Sakuraba H, Yoneda K, Yoshihara K, Satoh K, Kawakami R, Uto Y, Tsuge H, Takahashi K, Hori H, Ohshima T (2007) Sequential aldol condensation catalyzed by hyperthermophilic 2-deoxy-d-ribose-5-phosphate aldolase. Appl Environ Microbiol 73:7427–7434
Blanco RM, Terreros P, Munoz N, Serra E (2007) Ethanol improves lipase immobilization on a hydrophobic support. J Mol Catal B: Enzym 47:13–20
An HJ, Lee H-J, Jun S-H, Youn HS, Byoung CK, Kim K, Lee K-M, Oh M-K, Kim J (2011) Enzyme precipitate coatings of lipase on polymer nanofibers. Bioprocess Biosyst Eng 34:841–847
Parmar A, Kumar H, Marwaha S, Kennedy JF (2000) Advances in enzymatic transformation of penicillins to 6-aminopenicillanic acid (6-APA). Biotechnol Adv 18:289–301
Shen Q, Yang R, Hua X, Ye F, Zhang W, Zhao W (2011) Gelatin-templated biomimetic calcification for β-galactosidase immobilization. Process Biochem 46:1565–1571
Martinek K, Mozhaev VV (1991) Practice importance of enzyme stability. 2. Increase of enzyme stability by immobilization ans treatment with low-molecular-weight reagents. Pure Appl Chem 63:1533–1537
Cao L, Schmid RD (2006) Carrier-bound immobilized enzymes: principles, application and design. Wiley-VCH Verlag GmbH & Co, Weinheim
Wang AM, Zhou C, Liu MQ, Du ZQ, Zhu SM, Shen SB, Ouyang PK (2009) Enhancement of microwave-assisted covalent immobilization of penicillin acylase using macromolecular crowding and glycine quenching. J Biosci Bioeng 107:219–224
Wang A, Wang H, Zhu S, Zhou C, Du Z, Shen S (2008) An efficient immobilizing technique of penicillin acylase with combining mesocellular silica foams support and p-benzoquinone cross linker. Bioprocess Biosyst Eng 31:509–517
Kim YM, Chang YH, Choi NS, Kim Y, Song JJ, Kim JS (2009) Cloning, expression, and characterization of a new deoxyribose 5-phosphate aldolase from Yersinia sp EA015. Protein Expression Purif 68:196–200
Stumpf PK (1947) A colorimetric method for the determination of deoxyribonucleic acid. J Biol Chem 169:367–371
Wang A, Wang M, Wang Q, Chen F, Zhang F, Li H, Zeng Z, Xie T (2011) Stable and efficient immobilization technique of aldolase under consecutive microwave irradiation at low temperature. Bioresour Technol 102:469–474
Wang A, Zhang F, Chen F, Wang M, Li H, Zeng Z, Xie T, Chen Z (2011) A facile technique to prepare cross-linked enzyme aggregates using p-benzoquinone as cross-linking agent. Korean J Chem Eng 28:1090–1095
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 37:790–901
Dalal S, Kapoor M, Gupta MN (2007) Preparation and characterization of combi-CLEAs catalyzing multiple non-cascade reactions. J Mol Catal B: Enzym 44:128–132
Lopez-Gallego F, Betancor L, Hidalgo A, Alonso N, Fernandez-Lafuente R, Guisan JM (2005) Co-aggregation of enzymes and polyethyleneimine: a simple method to prepare stable and immobilized derivatives of glutaryl acylase. Biomacromolecules 6:1839–1842
Cao LQ (2005) Immobilised enzymes: science or art? Curr Opin Cell Biol 9:217–226
Drauz K, Waldmann H (2002) Enzyme catalysis in organic synthesis: a comprehensive handbook. Wiley-VCH, Weinheim
Yang L, Dordick JS, Garde S (2004) Hydration of enzyme in nonaqueous media is consistent with solvent dependence of its activity. Biophys J 87:812–821
Dordick JS (1992) Designing enzymes for use in organic solvents. Biotechnol Prog 8:259–267
Zaks A, Klibanov AM (1984) Enzymatic catalysis in organic media at 100 degrees C. Science 15:1249–1251
Kim Y-M, Choi N-S, Kim YO, Son DH, Chang Y-H, Song JJ, Kim JS (2010) Expression and characterization of a novel deoxyribose 5-phosphate aldolase from Paenibacillus sp. EA001. J Microbiol Biotechnol 20:995–1000
Jennewein S, Schürmann M, Wolberg M, Hilker I, Luiten R, Wubbolts M, Mink D (2006) Directed evolution of an industrial biocatalyst: 2-deoxy-d-ribose 5-phosphate aldolase. Biotechnol J 1:537–548
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (20906016, 21076053), the Technology Research and Development Program of Hangzhou (20090331N03), the Technology Research and Development Program of Zhejiang Province (2009C31086), the Special Foundation for New Researchers in Hangzhou Normal University (YS05203141) and the Foundation for Innovation of Science and Technology and Cultural Originality in Hangzhou Normal University (YS03201012).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, A., Gao, W., Zhang, F. et al. Amino acid-mediated aldolase immobilisation for enhanced catalysis and thermostability. Bioprocess Biosyst Eng 35, 857–863 (2012). https://doi.org/10.1007/s00449-011-0670-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0670-4