Abstract
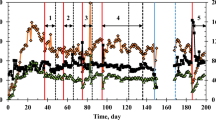
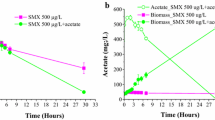
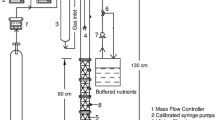
This work focuses on chloroform (CF) cometabolism by a butane-grown aerobic pure culture (Rhodococcus aetherovorans BCP1) in continuous-flow biofilm reactors. The goals were to obtain preliminary information on the feasibility of CF biodegradation by BCP1 in biofilm reactors and to evaluate the applicability of the pulsed injection of growth substrate and oxygen to biofilm reactors. The attached-cell tests were initially conducted in a 0.165-L bioreactor and, then, scaled-up to a 1.772-L bioreactor. Glass cylinders were utilized as biofilm carriers. The continuous supply of growth substrate (butane), which led to the attainment of the highest CF degradation rate (8.4 mgCF day−1 m −2biofilm surface ), was compared with four schedules of butane and oxygen pulsed feeding. The pulsed injection technique allowed the attainment of a ratio of CF mass degraded per unit mass of butane supplied equal to 0.16 mgCF mg −1butane , a value 4.4 times higher than that obtained with the continuous substrate supply. A procedure based on the utilization of integral mass balances and of average concentrations along the bioreactors resulted in a satisfactory match between the predicted and the experimental CF degradation performances, and can therefore be utilized to provide a guideline for optimizing the substrate pulsed injection schedule.






Similar content being viewed by others
Abbreviations
- b :
-
Endogenous decay coefficient (day−1)
- c i :
-
Aqueous phase concentration of compound i (g m−3; gprotein m−3 for the cells of BCP1)
- \( \bar{c}_{i} \) :
-
Average concentration of compound i along the bioreactor (g m−3; gprotein m−3 for the cells of BCP1)
- \( \bar{c}_{X}^{\prime \prime } \) :
-
Total cell mass in the bioreactor divided by the total interfacial area (gprotein m −2biofilm surface )
- \( \bar{c}_{X}^{\prime \prime \prime } \) :
-
Total cell mass in the bioreactor divided by the reactor volume (gprotein m −3reactor )
- D i :
-
Molecular diffusivity of compound i in water (m2 s−1)
- D L,i :
-
Longitudinal dispersion coefficient of compound i (m2 s−1)
- k CF,ac :
-
CF first-order constant for attached cells of BCP1 (m 3pore volume g −1protein day−1)
- k CF,sc :
-
CF first-order constant for suspended cells of BCP1 (m 3pore volume g −1protein day−1)
- K i,B or CF :
-
Constant of butane inhibition on CF degradation or CF inhibition on butane uptake (g m−3)
- K s,B or CF :
-
Butane or CF half-saturation constant (g m−3)
- L :
-
Column length (m)
- m CF :
-
Total CF mass in the bioreactor (g)
- m X :
-
Total cell mass in the bioreactor (gprotein)
- \( \dot{m}_{\text{B,inlet}} ,\dot{m}_{\text{CF,inlet}} \) :
-
Butane or CF mass rate entering the bioreactor (g day−1)
- n P :
-
Number of daily butane and oxygen pulses (–)
- Pe:
-
Peclet number, evaluated as \( {{vL} \mathord{\left/ {\vphantom {{vL} {D_{L,i} }}} \right. \kern-\nulldelimiterspace} {D_{L,i} }} \)(–)
- Q :
-
Flow rate (m3 day−1)
- q B or CF :
-
Butane or CF specific biodegradation rate (g g −1protein day−1)
- q max,B or CF :
-
Butane or CF maximum specific biodegradation rate (g g −1protein day−1)
- \( {{\bar{R}_{\text{CF}}^{\prime \prime } } \mathord{\left/ {\vphantom {{\bar{R}_{\text{CF}}^{\prime \prime } } {\bar{c}_{\text{CF}} }}} \right. \kern-\nulldelimiterspace} {\bar{c}_{\text{CF}} }} \) :
-
Total CF mass degraded daily, divided by the total interfacial area and by the average CF concentration along the reactor (Lpore volume m −2biofilm surface day−1)
- \( {{\bar{R}_{\text{CF}}^{\prime \prime \prime } } \mathord{\left/ {\vphantom {{\bar{R}_{\text{CF}}^{\prime \prime \prime } } {\bar{c}_{\text{CF}} }}} \right. \kern-\nulldelimiterspace} {\bar{c}_{\text{CF}} }} \) :
-
Total CF mass degraded daily, divided by the reactor volume and by the average CF concentration along the reactor (day−1)
- S biofilm :
-
Total surface of the biofilm carriers (m2)
- \( t_{\text{B,pulse}} \) :
-
Duration of each butane pulse (h)
- \( t_{\text{B}}^{*} \) :
-
Daily hours of butane feeding/24 (–)
- T c,CF :
-
CF transformation capacity (CF degraded per unit amount of cells inactivated by the toxic degradation products or by NADH depletion; gCF g −1protein )
- v :
-
Interstitial velocity (m h−1)
- Y B :
-
Cell growth yield on butane (gprotein g −1butane )
- α L :
-
Longitudinal dispersivity (m)
- ε biofilm :
-
Biofilm efficiency (–)
- η B , η CF :
-
Butane or CF conversion (–)
References
Chang HL, Alvarez-Cohen L (1996) Biodegradation of individual and multiple chlorinated aliphatic hydrocarbons by methane-oxidizing cultures. Appl Environ Microbiol 62:3371–3377
Anderson JE, McCarty PL (1996) Effect of three chlorinated ethenes on growth rates for a methanotrophic mixed culture. Environ Sci Technol 30:3517–3524
Morono Y, Unno H, Hori K (2006) Correlation of TCE cometabolism with growth characteristics on aromatic substrates in toluene-degrading bacteria. Biochem Eng J 31:173–179
Hori K, Mii J, Morono Y, Tanji Y, Unno H (2005) Kinetic analyses of trichloroethylene cometabolism by toluene-degrading bacteria harboring a tod homologous gene. Biochem Eng J 26:59–64
Ely RL, Williamson KJ, Hyman MR, Arp DJ (1997) Cometabolism of chlorinated solvents by nitrifying bacteria: kinetics, substrate interactions, toxicity effects and bacterial response. Biotechnol Bioeng 54:520–534
Kim Y, Arp D, Semprini L (2000) Chlorinated solvent cometabolism by butane-grown mixed culture. J Env Eng 126:934–942
Kim Y, Arp D, Semprini L (2002) Kinetic and inhibition studies for the aerobic cometabolism of 1, 1, 1-trichloroethane, 1, 1-dichloroethylene, and 1, 1-dichloroethane by a butane-grown mixed culture. Biotechnol Bioeng 80:498–508
Frascari D, Zannoni A, Fedi S, Pii Y, Zannoni D, Pinelli D, Nocentini M (2005) Aerobic cometabolism of chloroform by butane-grown microorganisms: long-term monitoring of depletion rates and isolation of a high-performing strain. Biodegradation 16:147–158
Frascari D, Pinelli D, Nocentini M, Pii Y, Fedi S, Zannoni D (2006) Chloroform degradation by butane-grown cells of Rhodococcus aetherovorans BCP1. Appl Microbiol Biotechnol 73:421–428
Frascari D, Zannoni A, Pinelli D, Nocentini M (2007) Chloroform aerobic cometabolism by butane-utilizing bacteria in bioaugmented and nonbioaugmented soil/groundwater microcosms. Proc Biochem 42:1218–1228
Semprini L, Dolan ME, Mathias MA, Hopkins GD, McCarty PL (2007) Laboratory, field, and modeling studies of bioaugmentation of butane-utilizing microorganisms for the in situ cometabolic treatment of 1, 1-dichloroethene, 1, 1-dichloroethane, and 1, 1, 1-trichloroethane. Adv Water Resour 30:1528–1546
Semprini L, Dolan ME, Hopkins GD, McCarty PL (2009) Bioaugmentation with butane-utilizing microorganisms to promote in situ cometabolic treatment of 1, 1, 1-trichloroethane and 1, 1-dichloroethene. J Contam Hydrol 103:157–167
Environmental Security Technology Certification Program (2001) Use of cometabolic air sparging to remediate chloroethene-contaminated groundwater aquifers Cost and performance report. US Department of Defense, Washington DC
Kim Y, Istok JD, Semprini L (2008) Single-well, gas-sparging tests for evaluating the in situ aerobic cometabolism of cis-1, 2-dichloroethene and trichloroethene. Chemosphere 71:1654–1664
Environment Canada (2001) Health Canada Priority substances list assessment report: Chloroform. ISBN 0-662-29247-2
USEPA (2007) Toxic Release Inventory Program. EPA, Washington
Delle Site A (2001) Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants: a review. J Phys Chem Ref Data 30:187–439
Mileva A, Sapundzhiev TS, Beschkov V (2008) Modeling 1, 2-dichloroethane biodegradation by Klebsiella oxytocava 8391 immobilized on granulated activated carbon. Bioprocess Biosyst Eng 31:75–85
Karamanev DG, Nikolov LN (1991) A comparison between the reaction rates in biofilm reactors and free suspended cells bioreactors. Bioprocess Biosyst Eng 6:127–130
Tziotzios G, Teliou M, Kaltsouni V, Lyberatos G, Vayenas DV (2005) Biological phenol removal using suspended growth and packed bed reactors. Biochem Eng J 26:65–71
Gandhi RK, Hopkins GD, Goltz MN, Gorelick SM, McCarty PL (2002) Full-scale demonstration of in situ cometabolic biodegradation of trichloroethylene in groundwater. 2: comprehensive analysis of field data using reactive transport modeling. Wat Resour Res 38:1040
Goltz MN, Bouwer EJ, Huang J (2001) Transport issues and bioremediation modeling for the in situ aerobic cometabolism of chlorinated solvents. Biodegradation 12:127–140
Hopkins GD, McCarty PL (1995) Field evaluation of in situ aerobic cometabolism of trichloroethylene and three dichloroethylene isomers using phenol and toluene as the primary substrates. Environ Sci Technol 29:1628–1637
McCarty PL, Goltz MN, Hopkins GD, Allan JP, Dolan ME, Kawakami BT, Carrothers TJ (1998) Full-scale evaluation of in situ cometabolic degradation of trichloroethylene in groundwater through toluene injection. Environ Sci Technol 32:88–100
Yuehuei HA, Friedman RJ (1997) Laboratory methods for studies of bacterial adhesion. J Microbiol Meth 30:141–152
Perry RH, Green DW, Maloney JO (1997) Perry’s chemical engineers’ handbook. McGraw-Hill, New York
Witherspoon PA, Saraf DN (1965) Diffusion of methane, ethane, propane, and n-butane in water from 25 to 43 °C. J Phys Chem 69:3752–3755
Smith LH, Kitanidis PK, McCarty PL (1997) Numerical modeling and uncertainties in rate coefficients for methane utilization and TCE cometabolism by a methane-oxidizing mixed culture. Biotechnol Bioeng 53:320–331
Alvarez-Cohen L, Speitel GE Jr (2001) Kinetics of aerobic cometabolism of chlorinated solvents. Biodegradation 12:105–126
Chang HL, Alvarez-Cohen L (1995) Model for the cometabolic biodegradation of chlorinated organics. Environ Sci Technol 29:2357–2367
Frascari D, Pinelli D, Nocentini M, Baleani E, Cappelletti M, Fedi S (2008) A kinetic study of chlorinated solvent cometabolic biodegradation by propane-grown Rhodococcus sp. PB1. Biochem Eng J 42:139–147
ISO (1995) ISO Guide 98-3: uncertainy of measurement—Part 3: guide to the expression of uncertainty in measurement
Berthouex PM, Brown LC (1994) Statistics for environmental engineers. Lewis, Boca Raton
Hamamura N, Page C, Long T, Semprini L, Arp DJ (1997) Chloroform cometabolism by butane-grown CF8, Pseudomonas butanovora and Mycobacterium vaccae job5 and methane-grown Methylosinus trichosporium OB3b. Appl Environ Microbiol 63:3607–3613
Chang WK, Criddle CS (1997) Experimental evaluation of a model for cometabolism: prediction of simultaneous degradation of trichloroethylene and methane by a methanotrophic mixed culture. Biotechnol Bioeng 54:491–501
Han NW, Bhakta J, Carbonell RG (1985) Longitudinal and lateral dispersion in packed beds: effect of column length and particle size distribution. AIChE J 31:277–288
Eidsath A, Carbonell RG, Whitaker S, Herrmann LR (1983) Dispersion in pulsed systems—III: comparison between theory and experiments for packed beds. Chem Eng Sci 38:1803–1816
Sun NZ (1996) Mathematical modeling of groundwater pollution. Springer, New York
Tchobanoglous G, Franklin LB, Stense HD (2003) Wastewater engineering: treatment and reuse. Metcalf & Eddy Inc, 4th edn. McGraw-Hill, Boston
Dolan ME, McCarty PL (1995) Methanotrophic chloroethene transformation capacities and 1, 1-dichloroethylene product toxicity. Environ Sci Technol 29:2741–2747
Alvarez-Cohen L, McCarty PL (1991) Two-stage dispersed-growth treatment of halogenated aliphatic compounds by cometabolism. Environ Sci Technol 25:1387–1393
Lackey LW, Phelps TJ, Bienkowski PR, White DC (1993) Biodegradation of chlorinated aliphatic hydrocarbon mixtures in a single pass packed bed reactor. Appl Biochem Biotechnol 39(40):701–713
Fayolle F, Le Roux F, Treboul C, Ballerini D (1995) TCE degradation by methanotrophic bacteria in a water-saturated sand column. In: Hinchee RE, Semprini L, Leeson A (eds) Bioremediation of chlorinated solvents. Battelle Press, Columbus
Lang MM, Roberts PV, Semprini L (1997) Model simulations in support of field scale design and operation of bioremediation based on cometabolic degradation. Ground Water 35:565–574
Roberts PV, Hopkins GD, Mackay DM, Semprini L (1990) A filed evaluation of in situ biodegradation of chlorinated ethenes: part 1, methodology and field site characterization. Ground Water 28:591–604
Rittman BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill, London
Das Chhaya, Chowdhury Ranjana, Bhattacharya Pinaki (2011) Experiments and three phase modelling of a biofilter for the removal of toluene and trichloroethylene. Bioprocess Biosyst Eng 34:447–458
Fang Y, Govind R (2008) A new Thiele’s modulus for the Monod biofilm model. Chin J Chem Eng 16:277–286
Semprini L, Hopkins GD, Roberts PV, Grbic-Galic D, McCarty PL (1991) A filed evaluation of in situ biodegradation of chlorinated ethenes: part 3, studies of competitive inhibition. Ground Water 29:239–250
Acknowledgments
Project co-funding by the Italian Ministry of University and Research through research grant PRIN 2008 “Novel processes for the sustainable remediation of groundwater contaminated by chlorinated compounds”, and by the European Commission under Grant Agreement no. 265946 (Minotaurus project, 7th FP) is acknowledged. The authors greatly acknowledge Luis Olarte, Michele Pugliese and Marco Ramos for their help in the experimental work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciavarelli, R., Cappelletti, M., Fedi, S. et al. Chloroform aerobic cometabolism by butane-growing Rhodococcus aetherovorans BCP1 in continuous-flow biofilm reactors. Bioprocess Biosyst Eng 35, 667–681 (2012). https://doi.org/10.1007/s00449-011-0647-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0647-3