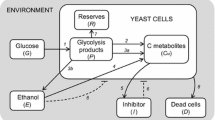
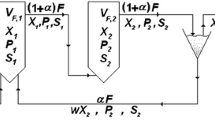
Abstract
Many mathematical models by researchers have been formulated for Saccharomyces cerevisiae which is the common yeast strain used in modern distilleries. A cybernetic model that can account for varying concentrations of glucose, ethanol and organic acids on yeast cell growth dynamics does not exist. A cybernetic model, consisting of 4 reactions and 11 metabolites simulating yeast metabolism, was developed. The effects of variables such as temperature, pH, organic acids, initial inoculum levels and initial glucose concentration were incorporated into the model. Further, substrate and product inhibitions were included. The model simulations over a range of variables agreed with hypothesized trends and to observations from other researchers. Simulations converged to expected results and exhibited continuity in predictions for all ranges of variables simulated. The cybernetic model did not exhibit instability under any conditions simulated.










Similar content being viewed by others
Abbreviations
- e i :
-
Concentration of ith enzyme (g/g cell mass) catalyzing reaction r i
- r i :
-
Reaction rate for ith pathway (1/s)
- t :
-
Time (s)
- C aa :
-
Concentration of acetic acid (g/L)
- C gy :
-
Concentration of glycerol (g/L)
- D :
-
Dilution rate (1/s)
- E :
-
Concentration of ethanol (g/L)
- EM:
-
Concentration of energy metabolite precursors (g/L)
- G :
-
Concentration of glucose (g/L)
- GA:
-
Concentration of glucoamylase (g/L)
- GP:
-
Concentration of glucose equivalent of dextrins (g/L)
- H :
-
Heaviside function
- K i :
-
Monod model constants (g/L)
- K gp :
-
Monod model constant in saccharification model (g/L)
- K g :
-
Product inhibition constant in saccharification model (g/L)
- K La :
-
Oxygen mass transfer coefficient (1/s)
- O :
-
Concentration of oxygen (g/L)
- O*:
-
Dissolved oxygen concentration limit (g/L)
- P ij :
-
Concentration for product produced in ith pathway and jth reaction
- pH:
-
Mash/fermenter pH
- SM:
-
Concentration of structural metabolite precursors (g/L)
- T :
-
Mash/fermenter temperature (°C)
- V max :
-
Rate constant in saccharification model (g/L-s)
- X :
-
Concentration of cell mass (g/L)
- Y i :
-
Yield coefficient for ith pathway
- \(\alpha_1,\;\alpha_2\) :
-
Coefficients for production of a unit cell mass from EM and SM in reaction r 3, respectively
- α*:
-
Constant intracellular enzyme synthesis rate (g/L s)
- β:
-
Constant intracellular enzyme breakdown rate (1/s)
- η 1 (1/s), η 2 (g/L s), η 3 (g/L s °C), η 4 (g/L s):
-
Assumed proportionality constants for including the environmental effects
- μ i :
-
Growth rate constant for ith pathway (1/s)
- ν:
-
Cybernetic variable controlling enzyme activity
- u :
-
Cybernetic variable controlling enzyme synthesis
- ϕ1 :
-
Coefficients for production of ethanol in reaction r 1
- ϕ2 :
-
Coefficients for consumption of oxygen in reaction r 4
- c:
-
Complementary pathway
- s:
-
Substitutable pathway
- n:
-
Number of reactions/alternative pathways
References
RFA (2010) Climate of opportunity: ethanol industry outlook 2006. http://www.ethanolrfa.org
Anuradha R, Suresh AK, Venkatesh KV (1999) Simultaneous saccharification and fermentation of starch to lactic acid. Process Biochem 35:367–375
Roy S, Gudi RD, Venkatesh KV, Shah SS (2001) Optimal control strategies for simultaneous saccharification and fermentation of starch. Process Biochem 36:713–722
Kroumov AD, Modenes AN, Tait MC (2006) Development of new unstructured model for simultaneous saccharification and fermentation of starch to ethanol by recombinant strain. Biochem Eng J 28:243–255
Srichuwonga S, Fujiwaraa M, Wanga X, Seyamaa T, Shiromaa R, Arakanea M, Mukojimab N, Tokuyasua K (2009) Simultaneous saccharification and fermentation (ssf) of very high gravity (vhg) potato mash for the production of ethanol. Biomass Bioenergy 33:890–898
Manikandan K, Viruthagiri T (2010) Kinetic and optimization studies on ethanol production from corn flour. Int J Chem Biol Eng 3:65–69
Murthy GS (2006) Development of a controller for fermentation in the dry grind corn process. Dissertation, Agricultural and Biological Engineering, University of Illinois, Urbana-Champaign, IL
Murthy GS, Singh V (2011) A dynamic optimal controller for control of fermentation processes. US Patent Office, US Patent 7,862,992.:171–180
Russel I (2003) Understanding yeast fundamentals. In: Jacques KA, Lyons TP, Kelsall DR (eds) The alcohol textbook: a reference for the beverage, fuel and industrial alcohol industries, 4th edn. Nottingham University Press, Nottingham, UK, pp 90, 96, 99, 100 and 102
Loureiro V, Van Uden N (1982) Effects of ethanol on the maximum temperature for growth of Saccharomyces cerevisiae: a model. Biotechnol Bioeng 24:1881–1884
Mager WH, Ferreira PM (1993) Stress response of yeast. Biochem Eng J 290:1–13
Thatipamala R, Rohani S, Hill GA (1992) Effects of high product and substrate inhibitions on the kinetics and biomass and product yields during ethanol batch fermentation. Biotechnol Bioeng 40:289–297
Narendranath NV, Hynes SH, Thomas KC, Ingledew WM (1997) Effects of Lactobacilli on yeast catalyzed ethanol fermentations. Appl Environ Microbiol 63:4158–4163
Narendranath NV, Thomas KC, Ingledew WM (2001) Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biotechnol 26:171–177
Bayrock DP, Ingledew WM (2004) Inhibition of yeast by lactic acid bacteria in continuous culture: nutrition depletion and/or acid toxicity?. J Ind Microbiol Biotechnol 31:362–368
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals, 2nd edn. McGraw Hill, Singapore
Straight JV, Ramakrishna D (1994) Cybernetic modeling and regulation of metabolic pathways. Growth on complementary nutrients. Biotechnol Progr 10:574–587
Jones KD, Kompala DS (1999) Cybernetic model of the growth dynamics of Saccharomyces cerevisiae in batch and continuous cultures. J Biotechnol 71:105–131
Verduyn C, Postma E, Scheffers WA, van Dijken JP (1990) Energetics of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol 136:405–412
Duarte NC, Herrgård MJ, Palsson BØ (2004) Reconstruction and validation of saccharomyces cerevisiae ind750, a fully compartmentalized genome-scale metabolic model. Genome Res 14:1298–1309
Nagodawithana TW, Catellano C, Steinkraus KH (1974) Effect of dissolved oxygen, temperature, initial cell count, and sugar concentration on viability of Saccharomyces cerevisiae in rapid fermentations. Appl Microbiol 28:383–391
Thomas KC, Hynes SH, Jones AM, Ingledew WM (1993) Production of fuel alcohol from wheat by vhg technology: Effect of sugar concentration and fermentation temperature. Appl Biochem Biotechnol 43:221–226
Verduyn C, Postma E, Scheffers WA, van Dijken JP (1990) Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol 136:395–403
AACC (2002) Method 44-15A. Approved methods of the AACC, 10th edn. The Association, St. Paul, MN
Pampulha ME, Loureiro-Dias MC (1989) Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl Microbiol Biotechnol 31:547–550
Liu Y, Liu Q, Tay J (2005) Initial conditions-dependent growth kinetics in microbial batch culture. Process Biochem 40:155–160
Author information
Authors and Affiliations
Corresponding author
A. Model summary
A. Model summary
A.1 Cybernetic control equations
A.2 Dynamic mass balance equations
Rights and permissions
About this article
Cite this article
Murthy, G.S., Johnston, D.B., Rausch, K.D. et al. A simultaneous saccharification and fermentation model for dynamic growth environments. Bioprocess Biosyst Eng 35, 519–534 (2012). https://doi.org/10.1007/s00449-011-0625-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0625-9