Abstract
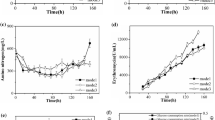
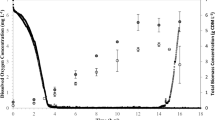
A metabolic network was constructed for the Acremonium chrysogenum cultivation fed with soybean oil. Metabolic flux analysis indicated that the shift from exponential growth to rapid cephalosporin C (CPC) formation was accompanied by 1.63- and 5-fold carbon flux enlargement in TCA cycle and glyoxylate by-pass, respectively. The flux via pentose phosphate pathway branch was little affected during the rapid CPC formation period; the contributory explanation was that 35.6% of NADPH was consumed in the dissimilation of fatty acids. Estimation of NADPH, ATP generation, and consumption demonstrated that, with soybean oil as carbon source in rapid CPC formation phase, the NADPH consumed in fatty acid catabolism was fourfold greater than that used in the CPC biosynthesis-relevant part; simultaneously, more than 90% energy spent was not directly related to the CPC formation. Therefore, the improvement of CPC production yield through optimization of the NADPH, ATP generation, and consumption was put forward.





Similar content being viewed by others
Abbreviations
- G-6-P:
-
Glucose-6-phosphate
- F-6-P:
-
Fructose-6-phosphate
- GAP:
-
Glyceraldehyde-3-phosphate
- 3-PG:
-
3-Phosphoglycerate
- Ru-5-P:
-
Ribulose-5-phosphate
- PEP:
-
Phosphoenolpyruvate
- PYR:
-
Pyruvate
- OAA:
-
Oxaloacetic acid
- ISOCIT:
-
Isocitrate
- α-KG:
-
α-Ketoglutarate
- α-AAA:
-
α-Amino adipinic acid
- CPC:
-
Cephalosporin C
- Metex :
-
Extracellular methionine
- ATP:
-
Adenosine triphosphate
- NADH:
-
Reduced diphosphopyridine nucleotide
- FADH2 :
-
Reduced flavin adenine dinucleotide
- NADPH:
-
Reduced nicotinamide adenine dinucleotide phosphate
References
Barber MS, Giesecke U, Reichert A, Minas W (2004) Industrial enzymatic production of cephalosporin-based β-lactams. Adv Biochem Eng Biotechnol 88:179–215
Zhou WC, Holzhauer-Rieger K, Dors M, Schugerl K (1992) Influence of medium composition on the cephalosporin C production with a highly productive strain Cephalosporium acremonium. J Biotechnol 23:315–329
Zhou WC, Holzhauer-Rieger K, Dors M, Schugerl K (1992) Influence of dissolved oxygen concentration on the biosynthesis of cephalosporin C. Enzyme Microb Technol 14:848–854
El-Sabbagh N, Harvey LM, McNeil B (2008) Effects of dissolved carbon dioxide on growth, nutrient consumption, cephalosporin C synthesis and morphology of Acremonium chrysogenum in batch cultures. Enzyme Microb Technol 42:315–324
Kim JH, Lim JS, Kim SW (2004) The improvement of cephalosporin C production by fed-batch culture of Cephalosporium acremonium M25 using rice oil. Biotechnol Bioprocess Eng 9:459–464
Lotfy WA (2007) The utilization of beet molasses as a novel carbon source for cephalosporin C production by Acremonium chrysogenum: optimization of process parameters through statistical experimental designs. Bioresour Technol 98:3491–3498
Behmer CJ, Demain AL (1983) Further studies on carbon catabolite regulation of β-lactam antibiotic synthesis in Cephalosporium acremonium. Curr Microbiol 8:107–114
Martin JF, Casqueiro J, Kosalkova K, Marcos AT, Gutierrz S (1999) Penicillin and cephalosporin biosynthesis: mechanism of carbon catabolite regulation of penicillin production. Antonie van Leeuwenhoek 75:21–31
Zhang JY, Wolfe S, Demain AL (1988) Phosphate repressible and inhibitable β-lactam synthetases in Cephalosporium acremonium strain C-10. Appl Microbiol Biotechnol 29:242–247
Tollnick C, Seidel G, Beyer M, Schugerl K (2004) Investigations of the production of cephalosporin C by Acremonium chrysogenum. Adv Biochem Eng Biotechnol 86:1–45
Schmitt EK, Hoff B, Kuck U (2004) Regulation of cephalosporin biosynthesis. Adv Biochem Eng Biotechnol 88:1–43
Holms H (1996) Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol Rev 19:85–116
Jorgensen H, Nielsen J, Villadsen J (1995) Metabolic flux distributions in Penicillium chrysogenum during fed-batch cultivations. Biotechnol Bioeng 46:117–131
Henriksen CM, Christensen LH, Villadsen J, Villadsen J (1996) Growth energetics and metabolic fluxes in continuous cultures of Penicillium chrysogenum. J Biotechnol 45:149–164
Zhang SL, Chu J, Zhuang YP (2004) A multi-scale study of industrial fermentation processes and their optimization. Adv Biochem Eng Biotechnol 87:97–150
Junker B, Mannn Z, Gailliot P, Byrne K, Wilson J (1998) Use of soybean oil and ammonium sulfate additions to optimize secondary metabolite production. Biotechnol Bioeng 60:580–588
Vallino JJ, Stephanopoulos G (1993) Metabolic flux distributions in Corynebacterium glutamicum during growth and lysine overproduction. Biotechnol Bioeng 41:633–646
Peacock LP, Ward J, Ratledge C, Dickinson FM, Andrew Ison (2003) How Streptomyces lividans uses oils and sugars as mixed substrates. Enzyme Microb Technol 32:157–166
Chu J, Li YR (2006) Modern concepts of industrial fermentation. Chemical Industry Press, Beijing
Hui YH (2004) Bailey’s industrial oil and fat products. China Light Industry Press, Beijing
Karaffa LK, Sandor E, Kozma J, Kubicek CP, Szentirmai A (1999) The role of the alternative respiratory pathway in the stimulation of cephalosporin C formation by soybean oil in Acremonium chrysogenum. Appl Microbiol Biotechnol 51:633–638
Seidel G, Tollnick C, Beyer M, Fahimi Y, Schugerl K (2002) Process engineering aspects of the production of cephalosporin C by Acremonium chrysogenum. Part I. Application of complex media. Process Biochem 38:229–239
Nasution U, van Gulik WM, Ras C, Proell A, Heijnen JJ (2008) A metabolome study of the steady-state relation between central metabolism, amino acid biosynthesis and penicillin production in Penicillium chrysogenum. Metab Eng 10:10–23
van Gulik WM, de Laat WTAM, Vinke JL, Heijnen JJ (2000) Application of metabolic flux analysis for the identification of metabolic bottlenecks in the biosynthesis of penicillin-G. Biotechnol Bioeng 68:602–618
Acknowledgments
This work was financially supported by a grant from the National High Technology Research and Development Program of China (863 Program), No. 2006AA020302, the Major State Basic Research Development Program of China (973 Program), No. 2007CB714303, National Key Technology Research and Development Program, No. 2008BAI63B01, 2007BAI26B02, and Shanghai Leading Academic Discipline Project, No. B505.
Author information
Authors and Affiliations
Corresponding authors
Appendix: Cellular biochemical reactions included in metabolic model
Appendix: Cellular biochemical reactions included in metabolic model
-
(1)
Uptake reactions
-
1.
glucose + ATP → G-6-P
-
2.
soybean oil → glycerol + fatty acids
-
3.
palmitic acid + ATP + 8CoASH → 8acetyl-CoA + 7FADH2 + 7NADH + AMP + 2Pi
-
4.
stearic acid + ATP + 9CoASH → 9acetyl-CoA + 8FADH2 + 8NADH + AMP + 2Pi
-
5.
arachidic acid + ATP + 10CoASH → 10acetyl-CoA + 9FADH2 + 9NADH + AMP + 2Pi
-
6.
oleic acid + ATP + 9CoASH → 9acetyl-CoA + 7FADH2 + 8NADH + AMP + 2Pi
-
7.
linoleic acid + ATP + 9CoASH + NADPH → 9acetyl-CoA + 6FADH2 + 8NADH + AMP + 2Pi
-
8.
linolenic acid + ATP + 9CoASH + NADPH → 9acetyl-CoA + 5FADH2 + 8NADH + AMP + 2Pi
-
9.
glycerol + ATP → GAP + NADH + ADP + Pi
-
(2)
Embden–Meyerhof–Parns pathway
-
10.
G-6-P → F-6-P
-
11.
F-6-P + ATP → 2GAP + ADP + Pi
-
12.
GAP → 3-PG + ATP + NADH
-
13.
3-PG → PEP
-
14.
PEP → PYR + ATP
-
(2X)
Gluconeogenesis
-
10X.
F-6-P → G-6-p
-
11X.
2GAP + H2O → F-6-P + Pi
-
12X.
3-PG + ATP + NADH → GAP
-
13X.
PEP → 3-PG
-
14.
PEP → PYR + ATP
-
15X.
OAA → PEP + CO2
-
(3)
TCA cycle
-
15.
PYR + CoASH → acetyl-CoA + CO2 + NADH
-
16.
OAA + acetyl-CoA → ISOCIT + CoASH
-
17.
ISOCIT → α-KG + CO2 + NADH
-
18.
α-KG + CoASH → succinyl-CoA + NADH + CO2
-
19.
succinyl-CoA → succinate + ATP + CoASH
-
20.
Succinate → malate + FADH2
-
21.
Malate → OAA + NADH
-
(4)
Glyoxylate bypass
-
22.
ISOCIT → succinate + glyoxylate
-
23.
glyoxylate + acetyl-CoA → malate + CoASH
-
(5)
Pentose phosphate pathway
-
24.
G-6-P + H2O+2NADP+ → Ru-5-P + 2NADPH + CO2 + 2H+
-
25.
3Ru-5-P→ 2F-6-P + GAP
-
(6)
Miscellaneous reactions and oxidative phosphorylation
-
26.
lactate → PYR + FADH2
-
27.
α-ketobutyrate + ATP + HSCoA → succinyl-CoA + NADH + ADP + Pi
-
28.
NADH+ 0.5O2→ 2.5ATP
-
29.
FADH2+ 0.5O2 → 1.5ATP
-
(7)
Biosynthesis of CPC
-
30.
2PYR + glutamate + NADPH → Valine + α-KG+CO2
-
31.
glutamate + acetyl-CoA → α-AAA + NADH + CO2 +HSCoA
-
32.
methionine + ATP → homocysteine + Adensine + PPi+Pi
-
33.
homocysteine + serine → cysteine +α-ketobutyrate+NH4 +
-
34.
serine + acetyl-CoA + SO4 2− + 4ATP + 4NADPH → cysteine + 4ADP + 4Pi + acetate
-
35.
α-AAA + cysteine + valine + 6ATP + 3O2 + 2α-KG + acetyl-CoA → CPC + 2succinate + 2CO2
-
(8)
Biosynthesis of amino acid and macromoleculars
-
36.
G-6-P → Biomass
-
37.
Ru-5-P → Biomass
-
38.
F-6-P → Biomass
-
39.
serine → Biomass
-
40.
PEP → Biomass
-
41.
PYR → Biomass
-
42.
acetyl-CoA → Biomass
-
43.
OAA → Biomass
-
44.
α-KG → Biomass
-
45.
α-AAA + glutamate + 2ATP + 2NADPH → lysine + α-KG + NADH + 2ADP + 2Pi
-
46.
lysine → Biomass
-
47.
methionine → Biomass
-
48.
valine → Biomass
Rights and permissions
About this article
Cite this article
Li, J., Yang, Y., Chu, J. et al. Quantitative metabolic flux analysis revealed uneconomical utilization of ATP and NADPH in Acremonium chrysogenum fed with soybean oil. Bioprocess Biosyst Eng 33, 1119–1129 (2010). https://doi.org/10.1007/s00449-010-0439-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-010-0439-1