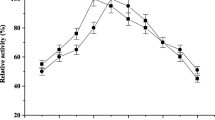
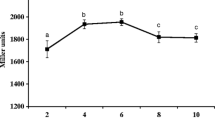
Abstract
A psychrotolerant yeast Guehomyces pullulans 17-1 was isolated from sea sediment in Antarctica. It was found that it could yield both extracellular and cell-bound β-galactosidase. After optimization of the medium and cultivation conditions, it was found that the yeast strain produced over 17.2 U mL−1 of β-galactosidase activity within 120 h at the flask level while the yeast strain produced over 25.3 U mL−1 of β-galactosidase activity within 144 h during the 2-L fermentation. This is the highest β-galactosidase activity produced by the wild type yeast strains reported so far. However, the optimal pH and temperature for the crude β-galactosidase were 4.0 and 50 °C, respectively. Lactose could be converted into glucose and galactose and a large amount of reducing sugar could be released from milk under catalysis of the yeast culture.







Similar content being viewed by others
References
Becerra M, Barolib B, Fadda AM, Méndez JB, Siso MIG (2001) Lactose bioconversion by calcium-alginate immobilization of Kluyveromyces lactis cells. Enz Microb Technol 29:506–512
Nakagawa T, Ikehata R, Uchino M (2006) Cold-active acid β-galactosidase activity of isolated psychrophilic-basidiomycetous yeast Guehomyces pullulans. Microbiol Resear 161:75–79
Sakai Y, Nakagawa T, Shimase M, Kato N (1998) Regulation and physiological role of the DAS1 gene encoding dihydroxyacetone synthase in the methylotrophic yeast Candida boidinii. J Bacteriol 180:5885–5890
Chi ZM, Ma CL, Wang P, Li HF (2007) Optimization of medium and cultivation conditions for alkaline protease production by the marine yeast Aureobasidium pullulans. Biores Technol 98:534–538
Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A (2003) Description of a new yeast species Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol 41:4695–4699
Felsenstein J (1995) PHYLIP (Phylogenetic Inference Package), Version 3.75. Distributed by author, Department of Genetics, University of Washington, Seattle, WA
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies on nucleotide sequences. J Mol Evol 2:87–90
Kurtzman CP, Fell JW (2000) The yeasts: a taxonomic study, 4th revised and enlarged edition. Elsevier, Amsterdam, pp 1–525
Nakagawa T, Fujimoto Y, Uchino M, Miyaji T, Takano K, Tomizuka N (2003) Isolation and characterization of psychrotrophs producing cold-active β-galactosidase. Lett Appl Microbiol 37:154–157
Spiro RG (1966) Analysis of sugars found in glycoproteins. Meth Enzymol 8:3–26
Gong F, Sheng J, Chi ZM, Li J (2007) Inulinase production by a marine yeast Pichia guilliermondii and inulin hydrolysis by the crude inulinase. J Ind Microbiol Biotechnol 34:179–185
Gerday C (2000) Cold-adapted enzymes: from fundamentals to biotechnology. TBTECH 18:103–107
Zhang ML, Liu WQ, Xiong H, Chen WH, Xiao F, Jian SQ (2006) Studies on the optimization for lactase production by Kluyveromyces lactis. Food Sci 27:428–431
Ding Q, Jiang YL, Shao JY (2001) The selection of optimal medium and condition for the production of lactase by yeast Kluyvermyces fragilis. J Zhe Jiang Univ. (Medical Sciences) l30:49–51
Majid HAMA, Tan SH, Gao XD, Wu WT (1999) Fermentation studies on yeast producing high level of lactase. J Chin Pharmaceut Univ 30:392–395
Konsoula Z, Liakopoulou-Kyriakides M (2007) Co-production of α-amylase and β-galactosidase by Bacillus subtilis in complex organic substrates. Biores Technol 98:150–157
Sener N, Kılıc D, O¨zbek BA (2006) A modelling study on milk lactose hydrolysis and β-galactosidase stability under sonication. Proc Biochem 41:1493–1500
Itoh T, Suzuki M, Adachi S (1982) Production and characterization of β-galactosidase from lactose-fermenting yeasts. Agric Biol Chem 46:899–904
Acknowledgments
This work was supported by National Infrastructure of Natural Resources for Science 386 and Technology Program of China (No. 2005DKA21209).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, C., Chi, Z., Li, J. et al. β-Galactosidase production by the psychrotolerant yeast Guehomyces pullulans 17-1 isolated from sea sediment in Antarctica and lactose hydrolysis. Bioprocess Biosyst Eng 33, 1025–1031 (2010). https://doi.org/10.1007/s00449-010-0427-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-010-0427-5