Abstract
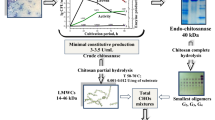
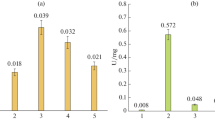
The products of chitosan hydrolysis are chitooligosaccharides and are used mainly for medical applications due to their specific biological activities. The objective of this study was to detect and identify the products of enzymatic hydrolysis of chitosan (dimers to hexamers) using a crude extract of chitosanolytic enzymes produced by the fungus Metarhizium anisopliae. These fungus was able to produce, during 48 h cultivation in a medium containing chitosan, chitooligosaccharides ranging from dimers, trimers, tetramers and pentamers at concentrations 0.2, 0.19, 0.06, 0.04 mg/mL, respectively, and the enzymatic activity was 2.5 U/L. Using the crude enzyme extract for chitosan hydrolysis, we detected the presence of dimers to hexamers at hydrolysis times of 10, 20, 30, 40, 50 and 60 min of enzymatic reaction, but the yields were higher at 10 min (54%). The hexamers was obtained only with 30 min of reaction with concentration of 0.004 mg/mL.





Similar content being viewed by others
References
Majeti NV, Kumar R (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27
Lee H-W, Choi J-W, Han D-P, Lee N-W, Park S-L, Yi D-H (1996) Identification and production of constitutive chitosanase from Bacillus sp. HW-002. J Microbiol Biotechnol 6(1):12–18
Kumirska J, Weinhold MX, Sauvageau JCM, Thöming J, Kaczynski Z, Stepnowski P (2009) Determination of the pattern of acetylation of low-molecular-weight chitosan used in biomedical applications. J Pharm Biom Anal 50:587–590
Chirkov SN (2002) The antiviral activity of chitosan (review). Appl Biochem Microbiol 38(1):1–8
Shahidi F, Synowiecki J (1991) Isolation and characterization of nutrients and value-added products from snow crab (Chinoecetes opilio) and shrimp (Pandalus borealis) processing discards. J Agric Food Chem 39:1527–1532
Agulló E, Rodriguez MS, Ramos V, Albertengo L (2003) Present and future role of chitin and chitosan in food. Macromol Biosci 3:521–530
Peniche-Covas C, Alvarez LW, Arguelles-Monal W (1992) The adsorption of mercuric ions by chitosan. J Appl Polym Sci 46:1147–1150
Nomanbhay SM, Palanisamy K (2005) Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. J Biotechnol 8(1):43–53
Pelletier A, Sygusch J (1990) Purification and characterization of three chitosanase activities from Bacillus megaterium P1. Appl Environ Microbiol 56:844–848
Somoshekar D, Joseph R (1996) Chitosanases—properties and applications: a review. Bioresour Technol 55:35–55
Zhou W, Yuan H, Wang J, Yao J (2008) Production, purification and characterization of chitosanase produced by Gongronella sp. JG. Lett Appl Microbiol 46:49–54
Price JS, Storck R (1975) Production, purification and characterization of an extracellular chitosanase from Streptomyces. J Bacteriol 124(3):1574–1585
Alfonos C, Martines M, Reyes F (1992) Purification and properties of two endochitosanase from Mucor rouxii implication on its cell wall degradation. FEMS Microbiol Lett 95:187–194
Kim P, Kang T, Chung K, Kim I, Chung K (2004) Purification of a constitutive chitosanase produced by Bacillus sp. MET 1299 with cloning and expression of the gene. FEMS Microbiol Lett 240:31–39
Valadares-Iglis MC, Peberdy JF (1997) Location of chitinolytic enzymes in protoplasts and whole cells of entomopathogenic fungus Metarhizium anisopliae. Mycol Res 101(11):1393–1396
Rombach M, Humber RA, Evans HCC (1987) Metarhizium album, a fungal pathogen of leaf and planthoppers of rice. Trans Br Mycol Soc 88:451–459
St Leger RJ, Cooper RM, Chamley AK (1986) Cuticle-degrading enzymes of entomopathogenic fungi: regulation of production of chitinolytic enzymes. J Gen Microbiol 132:1509–1517
Gupta SC, Leathers TD, El-Sayed GN, Ignoffo CM (1991) Production of degradative enzyme by Metarhizium anisopliae during growth on defined media and insect cuticle. Exp Mycol 15:310–315
Kim S-K, Rajapakse N (2005) Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydr Polym 62(4):357368
Hirano S, Nagao N (1989) Effects of chitosan, pectic acid lysozyme and chitinase on the growth of several phytopathogens. Agric Biol Chem 53:3065–3066
Jeon YJ, Kim SK (2000) Production of chitooligosaccharides using ultrafiltration membrane reactor and their antibacterial activity. Carboydr Polym 41:133–141
Jeon YJ, Kim SK (2000) Continuous production of chitooligosaccharides using a dual reactor system. Process Biochem 35:623–632
Jeon YJ, Park PJ, Kim SK (2001) Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carboydr Polym 44:71–76
Jeon YJ, Kim SK (2002) Antitumor activity of chitosan oligosaccharides produced in ultra filtration membrane reactor system. J Microbiol Biotech 12:503–507
Nam MY, Shon YH, Kim SK, Kim CH, Nam KS (1999) Inhibitory effect of chitosan oligosaccharides on the growth of tumor cells. J Chitin Chitosan 4:184–185
Suzuki K, Mikami T, Okawa Y, Tokoro A, Suzuki S, Suzuki M (1986) Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr Res 151:403–408
Yabuki M, Hirano M, Ando A, Fujii T, Amemiya Y (1987) Isolation and characterization of a chitosan degrading bacterium and formation of chitosananse by the isolate. Tech Bull Fac Hort Chiba Univ 39:23–27
Nahar P, Ghormade V, Deshpande MV (2004) The extracellular constitutive production of chitin deacetylase in Metarhizium anisopliae: possible edge to entomopathogenic fungi in the biological control of insect pests. J Inv Pathol 85:80–88
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Dou J, Tan C, Du Y, Bai X, Wang K, Ma X (2007) Effects of chitooligosaccharides on rabbit neutrophils in vitro. Carbohydr Polym 69(2):209–213
Liang T-W, Chen Y-J, Yen Y-H, Wang S-L (2007) The antitumor activity of the hydrolysates of chitinous materials hydrolyzed by crude enzyme from Bacillus amyloliquefaciens V656. Process Biochem 42(4):527–534
Chen X, Xia W, Yu X (2005) Purification and characterization of two types of chitosanase from Aspergillus sp. CJ22–326. Food Res Inter 38(3):315–322
Gooday GW, Zhu W-Y, O’Donnel RW (1992) What are the roles of chitinases in the growing fungus? Lett Appl Microbiol 100:387–391
Choi YJ, Kim EJ, Piao Z, Yun YC, Shim YC (2004) Purification and characterization of chitosanase from Bacillus sp. Stran KCTC 0377BP and its application for the production of chitosan oligosaccharides. Appl Environ Microbiol 70(8):4522–4531
Roncal T, Oviedo A, Armentia IL, Fernández L, Villarán (2007) High yield production of monomer-free chitosan oligosaccharides by pepsin catalyzed hydrolysis of a high deacetylation degree chitosan. Carboydr Res 342:2750–2756
Sashiwa H, Fujishima S, Yamano N, Kawasaki N, Nakayama A, Muraki E, Sukwattanasinitt M, Pichyangkura R, Aiba S-I (2003) Enzymatic production of N-acetyl-d-glucosamine from chitin: degradation study of N-acetylchitooligosaccharide and the effect of mixing of crude enzymes. Carboydr Polym 51:391–395
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10:37–51
Kuroiwa T, Ichikawa S, Hiruta O, Sato S, Mukataka S (2002) Factors affecting the composition of oligosaccharides produced in chitosan hydrolysis using immobilized chitosanases. Biotechnol Prog 18:969–974
Kuroiwa T, Sosaku I, Sato S, Mukataka S (2003) Improvement of yield of physiologically active oligosaccharides in continuous hydrolysis of chitosan using immobilized chitosanases. Biotechnol Bioeng 84(1):121–127
Kuroiwa T, Noguchi Y, Nakajima M, Sato S, Mukataka S, Ichikawa S (2008) Production of chitosan oligosacchades using chitosanase immobilized on amylose-coated magnetic nanoparticles. Process Biochem 43:62–69
Ming M, Kuroiwa T, Ichikawa S, Sato S, Mukataka S (2006) Production of chitosan oligosaccharides by chitosanase directly immobilized on an agar gel-coated multidisc impeller. Biochem Eng J 28(3):289–294
Acknowledgments
The authors thank Financiadora de Estudos e Projetos (FINEP), Conselho Nacional de Desenvolvimentos Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for support that made this work possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Assis, C.F., Araújo, N.K., Pagnoncelli, M.G.B. et al. Chitooligosaccharides enzymatic production by Metarhizium anisopliae . Bioprocess Biosyst Eng 33, 893–899 (2010). https://doi.org/10.1007/s00449-010-0412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-010-0412-z