Abstract
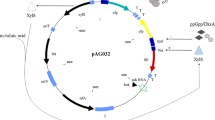
Due to the lack of appropriate sensors for monitoring changes of Escherichia coli cells and the huge complexity of cellular systems, many of the present protein production processes are still far from optimal. Aiming at maximal exploitation of the host cell, enhanced knowledge of cellular reactions related to recombinant protein expression is required. Current methods like DNA microarrays and 2-D-electrophoresis enable the acquisition of transcriptional and translational activity shifts in stress situations like heat shock, general stress response, nutrient limitation, and stress caused by overexpression of heterologous proteins. However, these techniques and data processing are time consuming, therefore, the goal is to create new on-line systems such as stress promoter GFP fusions to monitor metabolic alterations. The fluorescence signal of expressed GFP can be measured by 2-D-multi-wavelength fluorescence spectroscopy, thereby allowing non-invasive on-line in vivo monitoring. Results of efficient stress monitoring approaches in ongoing protein production process are presented.





Similar content being viewed by others
References
Demain AL (2000) Microbial biotechnology. Trends Biotechnol 18(1):26–31
Blattner FR, Pukett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y (1997) The complete genome sequence of Escherichia coli K-12. Science 277(5331):1453–1474
Bentley WE, Mirjalili N, Andersen DC, Davis RH, Kompala DS (1990) Plasmid encoded protein: the principal factor in the “metabolic burden” associated with recombinant bacteria. Biotechnol Bioeng 35:668–681
Wick LM, Egli T (2004) Molecular components of physiological stress responses in Escherichia coli. Adv Biochem Eng Biochnol 89:1–45
Cashel M, Rudd KE (1987) The stringent response. In: Neidhardt FC, Ingraham JL, Broosk Low K, Magasanik B, Schächter M, Umbarger H (eds) Escherichia coli and Salmonella typhimurim: cellular and molecular biology. American Society of Microbiology, Washington DC pp 1410–1438
Hoffmann F, Rinas U (2004) Stress induced by recombinant protein production in Escherichia coli. Adv Biochem Eng Biotechnol 89:73–92
Marose S, Lindemann C, Scheper T (1998) Two-dimensional fluorescence spectroscopy: a new tool for on-line bioprocess monitoring. Biotechnol Prog 14(1):63–74
Clementschitsch F, Kern J, Pötschacher F, Bayer K (2005) Sensor combination and chemometric modelling for improved process monitoring in recombinant E. coli fed-batch cultivations. J Biotechnol 120:183–196
Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as marker for gene expression. Science 263(5148):802–805
Cha HJ, Srivastava R, Vakhaira VN, Rao G, BentleyWE (1999) Green fluorescent protein as a non invasive probe in resting Escherichia coli cells. Appl Environ Microbiol. 65(2):409–414
Cormack BP, Valdivia RH, Falkow S (1996) FACS-optimized mutants of green fluorescent protein (GFP). Gene 173(1):33–38
Reischer H, Schotola I, Striedner G, Pötschacher F, Bayer K (2004) Evaluation of the GFP signal and its aptitude for novel on-line monitoring strategies of recombinant fermentation processes. J Biotechnol 108:115–125
Chang DE, Smalley DJ, Conway T (2002) Gene expression profiling of Escherichia coli growth transitions: an expanded stringent response model. Mol Microbiol 45(2):289–306
Yoon SH, Han MJ, Lee SY, Jeong KJ, Yoo JS (2003) Combined transcriptome and proteome analysis of Escherichia coli during high cell density culture. Biotechnol Bioeng 81(7):753–767
Haddadin FT, Harcum SW (2005) Transcriptome profiles for high-cell-density recombinant and wild-type Escherichia coli. Biotechnol Bioeng 90(2):127–153
Vostiar I, Tkac J, Mandenius CF (2004) Off-line monitoring of bacterial stress response during recombinant protein production using optical biosensors. J Biotechnol 111:191–201
Petersson L, Carrio MM, Vera A, Villaverde A (2004) The impact of dnaKJ overexpression on recombinant protein solubility results from antagonistic effects on the control of protein quality. Biotechnol Lett 26(7):595–601
Fürste JP, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E (1986) Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48(1):119–131
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. PNAS 97(12):6640–6645
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Habor Laboratory Press, ISBN:0879695765
Studier FW (1991) Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Bio 219(1):37–44
Moffatt BA, Studier FW. (1988) Entry of bacteriophage T7 DNA into the cell and escape from host restriction. J Bacteriol 170(5):2095–2105
DeBoy RT, Craig NL (2000) Target site selection by Tn7: attTn7 transcription and target activity. J Bacteriol 182(11):3310–3313
Phillips GJ (1998) New cloning vectors with temperature-sensitive replication. Plasmid 41(1):78–81
Porstmann T, Wietschke R, Grunow R, Jahn S, Porstmann B, Schmechta H, Pergande M, Von Baehr R (1990) Production and characterization of monoclonal antibodies against human Cu/Zn superoxide dismutase and the establishment of a super-rapid enzyme-linked immunosorbent assay (SURALISA). J Immunol Methods 127(1):1–10
Cserjan-Puschmann M, Kramer W, Duerrschmid E, Striedner G, Bayer K (1999) Metabolic approaches for the optimisation of recombinant fermentation processes. PNAS 53(1):43–50
Glick BR (1995) Metabolic load and heterologous gene expression. Biotechnol Adv 13(2):247–261
Acknowledgments
This work has been supported by the Austrian Center of Biopharmaceutical Technology (ACBT, http://www.acbt.at). We acknowledge this support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemecek, S., Marisch, K., Juric, R. et al. Design of transcriptional fusions of stress sensitive promoters and GFP to monitor the overburden of Escherichia coli hosts during recombinant protein production. Bioprocess Biosyst Eng 31, 47–53 (2008). https://doi.org/10.1007/s00449-007-0143-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-007-0143-y