Abstract
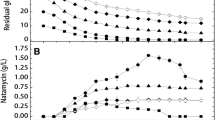
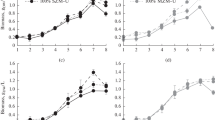
Spiramycin production by Streptomyces ambofaciens Sp181110 with glucose as the carbon source was studied under a controlled nutritional environment. In a batch culture, the glucose excess after ammonium depletion led to pyruvate and α-ketoglutarate accumulation. 85 mg/l of spiramycin were produced in less than 70 h during the stationary and maintenance phase on these acids after glucose exhaustion. Fed-batch strategy was designed to study spiramycin production without by-product formation and glucose accumulation. In these conditions, up to 150 mg/l were produced in less than 80 h during the stationary phase on glucose. The antibiotic titre was found independent of the glucose feeding under carbon limitation and the importance of putative intracellular reserves formed after nutrient exhaustion was suggested. Besides, spiramycin production was not inhibited by the limiting flux of glucose.





Similar content being viewed by others
References
Thomson CJ, Power E, Ruebsamen-Waigmann H, Labischinski H (2004) Curr Opin Microbiol 7(5):445–450
Demain AL (2000) Biotechnol Adv 18(6):499–514
Pinnert-Sindico S (1954) Ann Inst Pasteur (Paris) 87:702–707
Omura S, Takeshima H, Nakagawa A, Miyazawa J, Piriou F, Lukacs G (1977) Biochemistry 16(13):2860–2866
Blondelet-Rouault MH, Dominguez H, Darbon-Rongere E, Gerbaud C, Gondran A, Karray F, Lacroix P, Oestreicher-Mermet-Bouvier N, Pernodet JL, Tuphile K (2004) PCT Patent Application WO2004/033689 A2
Tang L, Zhang YX, Hutchinson CR (1994) J Bacteriol 176(19):6107–6119
Omura S, Kitao C, Hamada H, Ikeda H (1979) Chem Pharm Bull (Tokyo) 27(1):176–182
Jin ZH, Cen PL (2004) J Zhejiang Univ Sci 5(6):689–695
Lounes A, Lebrihi A, Benslimane C, Lefebvre G, Germain P (1996) Appl Microbiol Biotechnol 45(1–2):204–211
Untrau-Taghian S, Lebrihi A, Germain P, Lefebvre G (1995) Can J Microbiol 41:157–162
Lounes A, Lebrihi A, Benslimane C, Lefebvre G, Germain P (1995) Curr Microbiol 31(5):304–311
Khaoua S, Lebrihi A, Laakel M, Schneider F, Germain P, Lefebvre G (1992) Appl Microbiol Biotechnol 36(6):763–767
Lounes A, Lebrihi A, Benslimane C, Lefebvre G, Germain P (1996) Process Biochem 31(1):13–20
Lebrihi A, Lamsaif D, Lefebvre G, Germain P (1992) Appl Microbiol Biotechnol 37(3):382–387
Lounes A, Lebrihi A, Benslimane C, Lefebvre G, Germain P (1995) Can J Microbiol 41(9):800–808
Laakel M, Lebrihi A, Khaoua S, Schneider F, Lefebvre G, Germain P (1994) Microbiology 140:1451–1456
Untrau S, Lebrihi A, Lefebvre G, Germain P (1994) Curr Microbiol 28:111–118
Untrau S, Lebrihi A, Germain P, Lefebvre G (1992) Curr Microbiol 25:313–318
Richardson MA, Kuhstoss S, Huber ML, Ford L, Godfrey O, Turner JR, Rao RN (1990) J Bacteriol 172(7):3790–3798
Geistlich M, Losick R, Turner JR, Rao RN (1992) Mol Microbiol 6(14):2019–2029
Pernodet JL, Alegre MT, Blondelet-Rouault MH, Guerineau M (1993) J Gen Microbiol 139(Pt 5):1003–1011
Martin JF, Demain AL (1980) Microbiol Rev 44(2):230–251
Egli T, Fiechter A (1981) J Gen Microbiol 123:365–369
Miller GL (1959) Anal Chem 31:426–429
Kuzdzal-Savoie S, Lebon F (1971) Tech lait 690:12–13
van der Heijden R, Heijnen J, Hellinga C, Romein B, Luyben K (1994) Biotechnol Bioeng 43:3–20
Roels JA (1983) Elsevier Biomedical Press, Amsterdam
Hodgson DA (2000) Adv Microb Physiol 42:47–238
Olukoshi ER, Packter NM (1994) Microbiology 140(4):931–943
Schauner C, Dary A, Lebrihi A, Leblond P, Decaris B, Germain P (1999) Appl Environ Microbiol 65(6):2730–2737
Pons MN, Drouin JF, Louvel L, Vanhoutte B, Vivier H, Germain P (1998) J Biotechnol 65(1):3–14
Acknowledgments
This study was supported by Sanofi-Aventis, Vitry sur Seine, France
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colombié, V., Bideaux, C., Goma, G. et al. Effects of glucose limitation on biomass and spiramycin production by Streptomyces ambofaciens. Bioprocess Biosyst Eng 28, 55–61 (2005). https://doi.org/10.1007/s00449-005-0015-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-005-0015-2