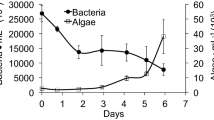
Abstract
Many marine planktonic ciliates retain functional chloroplasts from their photosynthetic prey and use them to incorporate inorganic carbon via photosynthesis. While this strategy provides the ciliates with carbon, little is known about their ability to incorporate major dissolved inorganic nutrients, such as nitrogen and phosphorus. Here, we studied how ciliates respond to different concentrations of dissolved inorganic nitrogen and phosphorus. Specifically, we tested the direct and indirect effects of nutrient availability on the ciliate Strombidium cf. basimorphum fed the cryptophyte prey Teleaulax amphioxeia. We assessed responses in the rates of growth, ingestion, photosynthesis, inorganic nutrient uptake, and excretion. Our results show that the prey changed its carbon content depending on the nutrient concentrations. Low inorganic nutrient concentrations increased S. cf. basimorphum growth and prey ingestion. The higher carbon content of the prey under these low nutrient conditions likely supported the growth of the ciliate, while the higher carbon:nutrient stoichiometry of the prey led to the higher ingestion rates. The low carbon content of the prey at high nutrient concentrations resulted in reduced growth of S. cf. basimorphum, which indicates that carbon acquired via photosynthesis in the ciliate cannot compensate for the ingestion of prey with low carbon content. In conclusion, our findings show S. cf. basimorphum is not able to utilize dissolved inorganic nitrogen and phosphorus for growth, and this species seems to be well adapted to exploit its prey when grown at low nutrient conditions.





Similar content being viewed by others
Data availability
All data produced for this study are provided in this manuscript.
Code availability
Not applicable.
References
Berman-Frank I, Dubinsky Z (1999) Balanced growth in aquatic plants: myth or reality? BioScience 29–37. https://doi.org/10.1525/bisi.1999.49.1.29
Boersma M, Aberle N, Hantzsche FM, Schoo KL, Wiltshire KH, Malzahn AM (2008) Nutritional limitation travels up the food chain. Int Rev Hydrobiol 93:479–488. https://doi.org/10.1002/iroh.200811066
Calbet A (2008) The trophic roles of micro zooplankton in marine systems. ICES J Mar Sci 65:325–331
Calbet A, Landry MR (2004) Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol Oceanogr 49:51–57. https://doi.org/10.4319/lo.2004.49.1.0051
Chrzanowski TH, Lukomski NC, Grover JP (2010) Element stoichiometry of a mixotrophic protist grown under varying resource conditions. J Eukaryot Microbiol 57:322–327
Dolan JR (1997) Phosphorus and ammonia excretion by planktonic protists. Mar Geol 139:109–122
Falkowski PG, Barber RT, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281:200–206. https://doi.org/10.1126/science.281.5374.200
Ferrier-Pages C, Rassoulzadegan F (1994) N remineralization in planktonic protozoa. Limnol Oceanogr 39:411–419. https://doi.org/10.4319/lo.1994.39.2.0411
Flynn KJ, Mitra A, Anestis K, Anschütz AA, Calbet A, Ferreira GD, Gypens N, Hansen PJ, John U, Martin JL, Mansour JS, Maselli M, Medić N, Norlin A, Not F, Pitta P, Romano F, Saiz E, Schneider LK, Stolte W, Traboni C (2019) Mixotrophic protists and a new paradigm for marine ecology: where does plankton research go now? Journal of Plankton Research 00. https://doi.org/10.1093/plankt/fbz026
Garcia NS, Sexton J, Riggins T, Brown J, Lomas MW, Martiny AC (2018) High variability in cellular stoichiometry of carbon, nitrogen, and phosphorus within classes of marine eukaryotic phytoplankton under sufficient nutrient conditions. Front Microbiol 9:1–10. https://doi.org/10.3389/fmicb.2018.00543
Geider RJ, La Roche J (2002) Redfield revisited: Variability of C:N: P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17. https://doi.org/10.1017/S0967026201003456
Ghyoot C, Flynn KJ, Mitra A, Lancelot C (2017a) Modeling plankton mixotrophy : a mechanistic model consistent with the shuter-type biochemical approach. Front Ecol Evol 5:78. https://doi.org/10.3389/fevo.2017.00078
Ghyoot C, Lancelot C, Flynn KJ, Mitra A, Gypens N (2017b) Introducing mixotrophy into a biogeochemical model describing an eutrophied coastal ecosystem: The Southern North Sea. Prog Oceanogr 157:1–11
Gifford D (1985) Laboratory culture of marine planktonic oligotrichs (Ciliophora, Oligotrichida). Mar Ecol Prog Ser 23:257–267. https://doi.org/10.3354/meps023257
Golz AL, Burian A, Winder M (2015) Stoichiometric regulation in micro- and mesozooplankton. J Plankton Res 37:293–305
Gruber DF, Tuorto S, Taghon GL (2009) Growth phase and elemental stoichiometry of bacterial prey influences ciliate grazing selectivity. J Eukaryot Microbiol 56:466–471
Guillard RR (1975) Culture of phytoplankton for feeding marine invertebrates. Springer, Culture of marine invertebrate animals Boston, MA, pp 29–60
Hansen PJ, Moldrup M, Tarangkoon W, Garcia-Cuetos L, Moestrup O (2012) Direct evidence for symbiont sequestration in the marine red tide ciliate Mesodinium rubrum. Aquat Microb Ecol 66:63–75. https://doi.org/10.3354/ame01559
Haraguchi L, Jakobsen HH, Lundholm N, Carstensen J (2018) Phytoplankton community dynamic: a driver for ciliate trophic strategies. Front Mar Sci 5:1–16. https://doi.org/10.3389/fmars.2018.00272
Heinbokel JF, Diego S, Jolla L (1978) Studies on the functional role of tintinnids in the Southern California Bight. I. Grazing and growth rates in laboratory cultures. Marine Biology 47, 189: 177–89.
Hessen DO, Elser JJ, Sterner RW, Urabe J (2013) Ecological stoichiometry: An elementary approach using basic principles. Limnol Oceanogr 58:2219–2236
Johnson MD, Beaudoin DJ (2019) The genetic diversity of plastids associated with mixotrophic oligotrich ciliates. 1–15. Limnol Oceanogr 64:2187–2201
Johnson MD, Oldach D, Delwiche CF, Stoecker DK (2007) Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature 445:426–428. https://doi.org/10.1038/nature05496
Johnson MD, Beaudoin DJ, Laza-Martinez A, Dyhrman ST, Fensin E, Lin S, Merculief A, Nagai S, Pompeu M, Setälä O, Stoecker DK (2016) The genetic diversity of Mesodinium and associated cryptophytes. Front Microbiol 7:1–16. https://doi.org/10.3389/fmicb.2016.02017
Katechakis A, Haseneder T, Kling R, Stibor H (2005) Mixotrophic versus photoautotrophic specialist algae as food for zooplankton: the light: nutrient hypothesis might not hold for mixotrophs. Limnol Oceanogr 50:1290–1299
Kim M, Park MG (2019) Unveiling the hidden genetic diversity and chloroplast type of marine benthic ciliate Mesodinium species. Scientific Reports 1–10. https://doi.org/10.1038/s41598
Koroleff F (1970) Information on techniques and methods for sea water analysis. Inter Lab Report 3:19–22
Lee BI, Kim SK, Kim JH, Kim HS, Kim JI, Shin W, Rho J, Yih W (2019) Intraspecific variations in macronutrient, amino acid, and fatty acid composition of mass-cultured Teleaulax amphioxeia ( Cryptophyceae ) strains. Algae 34:163–175
Liu W, Yi Z, Warren A, Al-rasheid KAS, J SAA, Lin X, Song W (2011) Taxonomy, morphology and molecular systematics of a new oligotrich ciliate, Williophrya maedai gen. nov., sp. nov., with redescriptions of Strombidium basimorphum and Pseudotontonia simplicidens (Protozoa, Ciliophora, Oligotrichia) Systematics and Biodiversity. 9: 247–258. https://doi.org/10.1080/14772000.2011.605812.
Malzahn AM, Hantzsche F, Schoo KL, Boersma M, Aberle N (2010) Differential effects of nutrient-limited primary production on primary, secondary or tertiary consumers. Oecologia 162(35–48):35–48. https://doi.org/10.1007/s00442-009-1458-y
Martin AJ, Montagnes DJ (1993) Winter ciliates in a British Columbian fjord: six new species and an analysis of ciliate putative prey. J Eukaryot Microbiol 40:535–549
Maselli M, Altenburger A, Stoecker DK, Hansen PJ (2020) Ecophysiological traits of mixotrophic Strombidium spp. Jouranl of Plankton Research 42:485–496. https://doi.org/10.1093/plankt/fbaa041
McManus GB, Schoener DM, Haberlandt K (2012) Chloroplast symbiosis in a marine ciliate: ecophysiology and the risks and rewards of hosting foreign organelles. Front Microbiol 3:1–9. https://doi.org/10.3389/fmicb.2012.00321
Mcmanus GB, Liu W, Cole RA, Biemesderfer D, Mydosh JL (2018) Strombidium rassoulzadegani : a model species for chloroplast retention in oligotrich ciliates. Front Mar Sci 5:1–11. https://doi.org/10.3389/fmars.2018.00205
Mitra A, Flynn KJ (2005) Predator-prey interactions: Is “ecological stoichiometry” sufficient when good food goes bad? J Plankton Res 27:393–399
Montagnes DJ (1996) Growth responses of planktonic ciliates in the genera Strobilidium and Strombidium. Mar Ecol Prog Ser 130:241–254. https://doi.org/10.3354/meps130241
Moorthi SD, Ptacnik R, Sanders RW, Fischer R, Busch M, Hillebrand H (2017) The functional role of planktonic mixotrophs in altering seston stoichiometry. Aquat Microb Ecol 79:235–245
Orsi WD, Wilken S, del Campo J, Heger T, James E, Richards TA, Keeling PJ, Worden AZ, Santoro AE (2018) Identifying protist consumers of photosynthetic picoeukaryotes in the surface ocean using stable isotope probing. Environ Microbiol 20:815–827. https://doi.org/10.1111/1462-2920.14018
Persson J, Fink P, Goto A, Hood JM, Jonas J, Kato S (2010) To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119:741–751
Putt M, Stoecker DK (1989) An experimentally determined carbon : volume ratio for marine “oligotrichous” ciliates from estuarine and coastal waters. Limonology Oceanogr 34:1097–1103
Redfield AC (1934) On the proportions of organic derivatives in sea water and their relation to the composition of plankton. University Press of Liverpool, Liverpool
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:230–221
Saikia SK, Nandi S (2010) C and P in aquatic food chain: A review on C: P stoichiometry and PUFA regulation. Knowl Manag Aquat Ecosyst 398:1–14. https://doi.org/10.1051/kmae/2010024
Schoener DM, McManus GB (2012) Plastid retention, use, and replacement in a kleptoplastidic ciliate. Aquat Microb Ecol 67:177–187. https://doi.org/10.3354/ame01601
Schoener DM, McManus GB (2017) Growth, grazing, and inorganic C and N uptake in a mixotrophic and a heterotrophic ciliate. J Plankton Res 39:379–391. https://doi.org/10.1093/plankt/fbx014
Siuda ANS, Dam HG (2010) Effects of omnivory and predator-prey elemental stoichiometry on planktonic trophic interactions. Limnol Oceanogr 55:2107–2116. https://doi.org/10.4319/lo.2010.55.5.2107
Smith M, Hansen PJ (2007) Interaction between Mesodinium rubrum and its prey: importance of prey concentration, irradiance and pH. Mar Ecol Prog Ser 338:61–70. https://doi.org/10.3354/meps338061
Solórzano L, Sharp JH (1980) Determination of total dissolved nitrogen in natural waters 1. Limnol Oceanogr 25:751–754
Stoecker DK, Silver MW, Michaels AE, Davis LH (1988) Obligate mixotrophy in Laboea strobila a ciliate which retains chloroplasts. Mar Biol 99:415–423. https://doi.org/10.1007/BF02112135848
Stoecker DK, Silver MW, Michaels AE, Davis LH (1989) Enslavement of algal chloroplasts by four Strombidium spp. (Ciliophora, Oligotrichida). Mar Microb Food Webs 3:79–100
Stoecker DK, Johnson MD, De Vargas C, Not F (2009) Acquired phototrophy in aquatic protists. Aquat Microb Ecol 57:279–310. https://doi.org/10.3354/ame01340
Taylor WD, Lean DRS (1981) Radiotracer experiments on phosphorus uptake and release by limnetic microzooplankton. Can J Fish Aquat Sci 38:1316–1321. https://doi.org/10.1139/f81-177
Yang J, Löder MGJ, Jiang Y, Wiltshire KH (2019) Are tintinnids picky grazers: Feeding experiments on a mixture of mixotrophic dinoflagellates and implications for red tide dynamics. Mar Pollut Bull 149:110488. https://doi.org/10.1016/j.marpolbul.2019.110488
Funding
This research was supported by EC MSCA-ITN 2019 funding to the project MixITiN (grant number 766327). The Carlsberg foundation funded the Flow cytometer (grant number 113501). Thanks to Nico Helmsing for the technical support regarding the C, N and P analyses.
Author information
Authors and Affiliations
Contributions
MM and PJH conceived and designed the experiments. MM performed the experiments. DVW analyzed the biomass samples. MM analyzed the data and drafted the manuscript; DVW and PJH contributed to write the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Maarten Boersma.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maselli, M., Van de Waal, D.B. & Hansen, P.J. Impacts of inorganic nutrients on the physiology of a mixoplanktonic ciliate and its cryptophyte prey. Oecologia 199, 41–52 (2022). https://doi.org/10.1007/s00442-022-05162-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-022-05162-3