Abstract
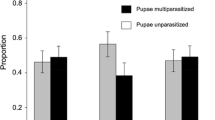
Interspecific competition for limited resources can drive ecological specialization and trait expression. Organisms released from intense competition may exploit a broader range of resources, but if reunited with stronger competitors, survivorship may depend on foraging behaviors that reduce competition. We compared the host selection behavior of the parasitoid Cotesia glomerata from two North American populations that differ in their association with Cotesia rubecula, a superior competitor. Both parasitoids originate from Europe and attack the imported cabbageworm (a.k.a. small cabbage white) Pieris rapae, but C. glomerata was introduced into North America almost a century before C. rubecula. After re-association in North America, C. rubecula has displaced C. glomerata in several regions, but not in other regions. Host selection was measured in female C. glomerata from Maryland (MD) where it coexists with C. rubecula, and in conspecifics from Colorado (CO) where C. rubecula is absent. Unparasitized and C. rubecula-parasitized P. rapae hosts were used in choice tests to examine whether C. glomerata host selection behavior differed based on the population’s association history with C. rubecula. We found that C. glomerata from MD had a higher likelihood of avoiding hosts parasitized by C. rubecula (and thus avoiding competition) than did wasps from CO. The ability of C. glomerata to avoid hosts parasitized by C. rubecula may facilitate coexistence in MD; whereas, the lack of discrimination in CO populations of C. glomerata naïve to C. rubecula could contribute to the displacement of C. glomerata were C. rubecula to enter the same habitat.



Similar content being viewed by others
References
Baldwin IT, Kessler A, Halitschke R (2002) Volatile signaling in plant-plant-herbivore interactions: what is real? Curr Opin Plant Biol 5:351–354
Benson J, Van Driesche RGV, Pasquale A, Elkinton J (2003) Introduced braconid parasitoids and range reduction of a native butterfly in New England. Biol Control 28:197–213
Biever KD (1992) Distribution and occurrence of Cotesia rubecula (Hymenoptera: Braconidae) a parasite of Artogeia rapae in Washington and Oregon. J Econ Entomol 85:739–742
Björklund H, Santangeli A, Blanchet FG, Huitu O, Lehtoranta H, Lindén H, Valkama J, Laaksonen T (2016) Intraguild predation and competition impacts on a subordinate predator. Oecologia 181:257–269
Britton JR, Ruiz-Navarro A, Verreycken H, Amat-Trigo F, Trullas SC (2018) Trophic consequences of introduced species: comparative impacts of increased interspecific versus intraspecific competitive interactions. Funct Ecol 32:486–495
Brodeur J, Geervliet JBF (1992) Host species affecting the performance of the larval Cotesia glomerata and Cotesia rubecula (Hymenoptera: Braconidae). Preference for host developmental stage of Pieris (Lepidoptera: Pieridae). Rijksuniv Fac Landbouwwet Gent 57:543–545
Brodeur J, Vet LEM (1995) Relationships between parasitoid host range and host defence: a comparative study of egg encapsulation in two related parasitoid species. Physiol Entomol 20:7–12
Brodeur J, Geervliet JBF, Vet LEM (1996) The role of host species, age and defensive behaviour on ovipositional decisions in a solitary specialist and gregarious generalist parasitoid (Cotesia species). Entomol Exp Appl 81:125–132
Brodeur J, Geervliet JBF, Vet LEM (1998) Effects of Pieris host species on life history parameters in a solitary specialist and gregarious generalist parasitoid (Cotesia species). Entomol Exp Appl 86:145–152
Brown JS, Kotler BP, Smith RJ, Wirtz WO II (1988) The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia 76:408–415
Chailleux A, Mohl EK, Alves MT, Messelink GJ, Desneux N (2014) Natural enemy-mediated indirect interactions among prey species: potential for enhancing biocontrol services in agroecosystems. Pest Manag Sci 70:1769–1779
Clausen CP (1978) Introduced parasites and predators of arthropod pests and weeds: a world review, Handbook 480. USDA. Agricultural Research Service, Washington DC
Cortesero AM, De Moraes CM, Stapel JO, Tumlinson JH, Lewis WJ (1997) Comparisons and contrasts in host-foraging strategies of two larval parasitoids with different degrees of host specificity. J Chem Ecol 23:1589–1606
Coss RG (1999) Effects of relaxed natural selection on the evolution of behavior. In: Foster SA, Endler JA (eds) Geographic variation in behavior: perspectives on evolutionary mechanisms. Oxford University Press, Oxford, pp 180–208
Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volkaert F (2001) Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc Natl Acad Sci 98:6256–6260
de Moraes CM, Mescher MC (2005) Intrinsic competition between larval parasitoids with different degrees of host specificity. Ecol Entomol 30:564–570
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Fatouros NE, van Loon JJA, Hordijk KA, Smid HM, Dicke M (2005) Herbivore-induced plant volatiles mediate in-flight host discrimination by parasitoids. J Chem Ecol 31:2033–2047
Fei M, Gols R, Harvey JA (2014) Seasonal phenology of interactions involving short-lived annual plants, multivoltine herbivore and its endoparasitoid wasp. J Anim Ecol 83:234–244
Fisher B (1961) A study in insect multiparasitism. J Exp Biol 38:267–275
Fullard JH, Ratcliffe JM, ter Hofstede H (2007) Neural evolution in the bat-free habitat of Tahiti: partial regression in an anti-predator auditory system. Biol Let 3:26–28
Gaines DN, Kok LT (1999) Impact of hyperparasitoids on Cotesia glomerata in southwestern Virginia. Biol Control 14:19–28
Gauthier N, Sanon A, Monge JP, Huignard J (1999) Interspecific relations between two sympatric species of Hymenoptera, Dinarmus basalis (Rond) and Eupelmus vuilleti (Crw.), ectoparasitoids of the bruchid Callosobruchus maculatus (F). J Insect Behav 12:399–413
Geervliet JBF, Brodeur L (1992) Host species affecting the performance of the larval parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: braconidae). Comparative suitability of three Pieris species (Lepidoptera: Pieridae). Rijksuniv Fac Landbouwwet Gent 57:547–550
Geervliet JBF, Vet LEM, Dicke M (1994) Volatiles from damaged plants as major cues in long-range host-searching by the specialist parasitoid Cotesia rubecula. Entomol Exp Appl 73:289–297
Geervliet JBF, Ariens S, Dicke M, Vet LEM (1998) Long-distance assessment of patch profitability through volatile infochemicals by the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: braconidae). Biol Control 11:113–121
Geervliet JBF, Verdel MSW, Snellen H, Schaub J, Dicke M, Vet LEM (2000) Coexistence and niche segregation by field populations of the parasitoids Cotesia glomerata and C. rubecula in the Netherlands: predicting field performance from laboratory data. Oecologia 124:55–63
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. University Press, Princeton
Gols R (2014) Direct and indirect chemical defences against insects in a multitrophic framework. Plant Cell Environ 37:1741–1752
Gols R, Ros VI, Ode PJ, Vyas D, Harvey JA (2019) Varying degree of physiological integration among host instars and their endoparasitoid affects stress-induced mortality. Entomol Exp Appl. https://doi.org/10.1111/eea.12765
Goubault M, Krespi L, Boivin G, Poinsot D, Nenon J, Cortesero AM (2004) Intraspecific variations in host discrimination behavior in the pupal parasitoid Pachycrepoideus vindemmiae Rondani (Hymenoptera: Pteromalidae). Environ Entomol 33:362–369
Grassel SM, Rachlow JL, Williams CJ (2015) Spatial interactions between sympatric carnivores: asymmetric avoidance of an intraguild predator. Ecol Evol 5:2762–2772
Harvey JA, Jervis MA, Gols R, Jiang N, Vet LEM (1999) Development of the parasitoid, Cotesia rubecula (Hymenoptera: Braconidae) in Pieris rapae and Pieris brassicae (Lepidoptera: Pieridae): evidence for host regulation. J Insect Physiol 1999:173–182
Harvey JA, Gols R, Strand MR (2009) Intrinsic competition and its effects on the survival and development of three species of endoparasitoid wasps. Entomol Exp Appl 130:238–248
Harvey JA, Poelman EH, Tanaka T (2013) Intrinsic inter- and intraspecific competition in parasitoid wasps. Annu Rev Entomol 58:331–351
Herlihy MV, Van Driesche RG, Abney MR, Brodeur J, Bryant AB, Casagrande RA, Delaney DA, Elkner TE, Fleischer SJ, Groves RL, Gruner DS, Harmon JP, Heimpel GE, Hemady K, Kuhar TP, Maund CM, Shelton AM, Seaman Z (2012) Distribution of Cotesia rubecula (Hymenoptera: Braconidae) and its displacement of Cotesia glomerata in Eastern North America. Fla Entomol 95:461–467
Ikawa T, Okabe H (1985) Regulation of egg number per host to maximize the reproductive success in the gregarious parasitoid, Apanteles glomeratus L. (Hymenoptera: Braconidae). Appl Entomol Zool 20:331–339
Ikawa T, Suzuki Y (1982) Ovipositional experience of the gregarious parasitoid, Apanteles glomeratus (Hymenoptera: Braconidae), influencing her discrimination of the host larvae, Pieris rapae crucivora. Appl Entomol Zool 17:119–126
Inouye DW (1978) Resource partitioning in bumblebees: experimental studies of foraging behavior. Ecology 59:672–678
Janssen A, van Alphen JJM, Sabelis MW, Bakker K (1995) Odour-mediated avoidance of competition in Drosophila parasitoids: the ghost of competition. Oikos 73:356–366
Kaser JM, Ode PJ (2016) Hidden risks and benefits of natural enemy-mediated indirect effects. Curr Opin Insect Sci 14:105–111
Kohler SL, McPeek MA (1989) Predation risk and the foraging behavior of competing stream insects. Ecology 70:1811–1825
Kusano H, Kitano H (1974) Studies on the ability of Apanteles glomeratus L. to discriminate parasitized host larvae, Pieris rapae crucivora, from intact ones. Kontyu Tokyo 42:358–364
Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, Coss RG, Donohue K, Foster SA (2012) Relaxed selection in the wild. Trends Ecol Evol 24:487–496
Laing JE, Corrigan J (1987) Intrinsic competition between the gregarious parasite, Cotesia glomeratus and the solitary parasite, Cotesia rubecula [Hymenoptera: Braconidae] for their host, Artogeia rapae [Lepidoptera: Pieridae]. Entomophaga 32:493–501
Laing JE, Levin DB (1982) A review of the biology and a bibliography of Apanteles glomeratus (L) (Hymenoptera: Braconidae). Biocontrol News Inf 3:7–23
Le Masurier AD, Waage JK (1993) A comparison of attack rates in a native and introduced population of the parasitoid Cotesia glomerata. Biocontrol Sci Tech 3:467–474
Magdaraog PM, Tanaka T, Harvey JA (2013) Inter- and intra-specific host discrimination in gregarious and solitary endoparasitoid wasps. Biocontrol 58:745–754
McDonald RC, Kok LT (1991) Hyperparasites attacking Cotesia glomerata (L.) and Cotesia rubecula (Marshall) (Hymenoptera: Braconidae) in southwestern Virginia. Biol Control 175:170–175
McDonald L, Kok RC (1992) Colonization and hyperparasitism of Cotesia rubecula (Hym.: Braconidae), a newly introduced parasite of Pieris rapae, in Virginia. Entomophaga 37:223–228
Milinski M, Heller R (1978) Influence of a predator on the optimal foraging behaviour of sticklebacks (Gasterosteus aculeatus L.). Nature 275:642–644
Murdoch WW, Briggs CJ, Nisbet RM (1996) Competitive displacement and biological control in parasitoids: a model. Am Nat 148:807–826
Nappi AJ (1975) Parasite encapsulation in insects. In: Maramoch K, Shope R (eds) Invertebrate immunity. Academic Press, New York, pp 293–326
Poelman EH, Gols R, Snoeren TAL, Muru D, Smid HM, Dicke M (2011) Indirect plant-mediated interactions among parasitoid larvae. Ecol Lett 14:670–676
Poelman EH, Buinsma M, Zhu F, Weldegergis BT, Boursault AE, Jongema Y, van Loon JJA, Vet LEM, Harvey JA, Dicke M (2012) Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biol 11:e1001435
Price PW (1972) Parasitoids utilizing the same host: adaptive nature of differences in size and form. Ecology 53:190–195
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Tagawa J (1992) Host discrimination by unmated individuals of the gregarious parasitoid wasp, Cotesia (= Apanteles) glomerata (Hymenoptera: Braconidae). Appl Entomol Zool 27:306–309
Tamò C, Roelfstra L, Guillaume S, Turlings TCJ (2006) Odour-mediated long-range avoidance of interspecific competition by a solitary endoparasitoid: a time-saving foraging strategy. J Anim Ecol 75:1091–1099
Thompson JN (1994) The coevolutionary process. University of Chicago Press, Chicago
Ueno T (1999) Multiparasitism and host feeding by solitary parasitoid wasps (Hymenoptera: Ichneumonidae) based on the pay-off from parasitized hosts. Ann Entomol Soc Am 92:601–608
van Alphen JJM, Visser ME (1990) Superparasitism as an adaptive strategy for insect parasitoids. Annu Rev Entomol 35:59–79
Van Baaren J, Boivin G (1998) Genotypic and kin discrimination in a solitary hymenopterous parasitoid: implications for speciation. Evol Ecol 12:523–534
van Dijken M, van Stratum P, van Alphen JJM (1992) Recognition of individual-specific marked parasitized hosts by the solitary parasitoid Epidinocarsis lopezi. Behav Ecol Sociobiol 30:77–82
Van Driesche RG, Nunn C (2002) Establishment of a Chinese strain of Cotesia rubecula (Hymenoptera: Braconidae) in the northeastern United States. Fla Entomol 85:386–388
Van Driesche RG, Nunn C, Pasquale A (2004) Life history pattern, host plants, and habitat as determinants of population survival of Pieris napi oleracea interacting with an introduced braconid parasitoid. Biol Control 29:278–287
Vet LEM, Sokolowski MB, MacDonald DE, Snellen H (1993) Responses of a generalist and a specialist parasitoid to drosophilid larval kairomones. J Insect Behav 6:615–624
Vos M, Hemerik L, Vet LEM (1998) Patch exploitation by the parasitoids Cotesia rubecula and Cotesia glomerata in multi-patch environments with different host distributions. J Anim Ecol 67:774–783
Wang X, Keller MA (2002) A comparison of the host-searching efficiency of two larval parasitoids of Plutella xylostella. Ecol Entomol 27:105–114
Weis JJ, Gray H, Heimpel GE (2016) High hyperparasitism of Cotesia rubecula (Hymenoptera: Braconidae) in Minnesota and Massachusetts. J Kansas Entomol Soc 89:385–389
Wilkinson ATS (1966) Apanteles rubecula Marsh and other parasites of Pieris rapae in British Columbia. J Econ Entomol 59:1012–1013
Willis EO (1966) Interspecific competition and the foraging behavior of plain-brown woodcreepers. Ecology 47:667–672
Wiskerke JSC, Vet LEM (1994) Foraging for solitarily and gregariously feeding caterpillars: a comparison of two related parasitoid species (Hymenoptera: Braconidae). J Insect Behav 7:585–603
Wold-Burkness SJ, Hutchison WD, Lee JC, Hines RL, Bolin PC, Heimpel GE (2005) A long-term survey of parasitoid species composition and parasitism of Trichoplusia ni (Lepidoptera: Noctuidae), Plutella xylostella (Lepidoptera: Plutellidae), and Pieris rapae (Lepidoptera: Pieridae) in Minnesota cabbage. J Entomol Sci 40:211–221
Zhu F, Weldegergis BT, Lhie B, Harvey JA, Dicke M, Poelman EH (2014) Body odors of parasitized caterpillars give away the presence of parasitoid larvae to their primary hyperparasitoid enemies. J Chem Ecol 40:986–995
Acknowledgements
We are grateful to personnel at Calvert Farm, Calvert Gift Farm, Flying Plow, Glade Link Farm and Gorman Farm for allowing us to collect insects from their crops; and to the staff at CSU ARDEC for helping to maintain our plants. Many thanks also to the staff at the Colorado State University Plant Growth Facilities for providing support in rearing plant and insect colonies. We thank H.L. Gray and J.J. Weis for their assistance in collecting C. rubecula near the University of Minnesota. Financial support was granted through the United States Department of Agriculture NIFA AFRI: 2014-67013- 2172 to P.J.O and G.E.H. Additional funding was provided to D.K.V. by the William M. Brown Professional Development Award, Sigma Xi (Grant no. G201603152036938) and the Colorado State University Graduate Degree Program in Ecology.
Author information
Authors and Affiliations
Contributions
DKV, PJO, and GEH conceived the ideas and designed methodology; DKV collected the data; DKV and PJO analyzed the data; DKV led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sven Bacher.
Enemy-free space plays a significant role in the evolutionary ecology of species, but most enemy-free space studies focus on trophic interactions (e.g., predator–prey, parasitoid–host). We know little about how a species is affected after escaping from interspecific competitors. This research is one of the few that shows behavioral changes in a weaker competitor following separation from its stronger competitor. These results enhance our understanding of how species may change when communities experience disruptions to established ecological relationships.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vyas, D.K., Harvey, J.A., Paul, R.L. et al. Ecological dissociation and re-association with a superior competitor alters host selection behavior in a parasitoid wasp. Oecologia 191, 261–270 (2019). https://doi.org/10.1007/s00442-019-04470-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04470-5