Abstract
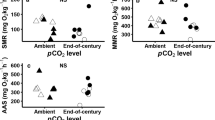
Increased levels of dissolved carbon dioxide (CO2) drive ocean acidification and have been predicted to increase the energy use of marine fishes via physiological and behavioural mechanisms. This notion is based on a theoretical framework suggesting that detrimental effects on energy use are caused by plasma acid–base disruption in response to hypercapnic acidosis, potentially in combination with a malfunction of the gamma aminobutyric acid type A (GABAA) receptors in the brain. However, the existing empirical evidence testing these effects primarily stems from studies that exposed fish to elevated CO2 for a few days and measured a small number of traits. We investigated a range of energetic traits in juvenile spiny chromis damselfish (Acanthochromis polyacanthus) over 3 months of acclimation to projected end-of-century CO2 levels (~ 1000 µatm). Somatic growth and otolith size and shape were unaffected by the CO2 treatment across 3 months of development in comparison with control fish (~ 420 µatm). Swimming activity during behavioural assays was initially higher in the elevated CO2 group, but this effect dissipated within ~ 25 min following handling. The transient higher activity of fish under elevated CO2 was not associated with a detectable difference in the rate of oxygen uptake nor was it mediated by GABAA neurotransmitter interference because treatment with a GABAA antagonist (gabazine) did not abolish the CO2 treatment effect. These findings contrast with several short-term studies by suggesting that end-of-century levels of CO2 may have negligible direct effects on the energetics of at least some species of fish.




Similar content being viewed by others
Data availability
The dataset supporting this manuscript is publicly archived in the repository figshare: https://doi.org/10.6084/m9.figshare.7965005, following best practices (Roche et al. 2015).
References
Bates D, Mächler M, Bolker BM, Walker SC (2014) lme4: Linear mixed-effects models using Eigen and S4 R package version 1.1-7
Baumann H, Talmage SC, Gobler CJ (2012) Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat Clim Change 2:38–41. https://doi.org/10.1038/nclimate1291
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 57:289–300
Bignami S, Enochs IC, Manzello DP, Sponaugle S, Cowen RK (2013a) Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc Natl Acad Sci USA 110:7366–7370. https://doi.org/10.1073/pnas.1301365110
Bignami S, Sponaugle S, Cowen RK (2013b) Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Glob Change Biol 19:996–1006. https://doi.org/10.1111/gcb.12133
Bignami S, Sponaugle S, Cowen RK (2014) Effects of ocean acidification on the larvae of a high-value pelagic fisheries species, mahi-mahi Coryphaena hippurus. Aquat Biol 21:249–260. https://doi.org/10.3354/ab00598
Boisclair D, Leggett W (1989) The importance of activity in bioenergetics models applied to actively foraging fishes. Can J Fish Aquat Sci 46:1859–1867
Branch TA, DeJoseph BM, Ray LJ, Wagner CA (2013) Impacts of ocean acidification on marine seafood. Trends Ecol Evol 28:178–186
Brauner C, Baker D (2009) Patterns of acid–base regulation during exposure to hypercarbia in fishes. In: Glass M, Wood S (eds) Cardio-respiratory control in vertebrates. Springer, Berlin, pp 43–63
Browman HI (2016) Applying organized scepticism to ocean acidification research. ICES J Mar Sci 73:529–536
Chambers R, Candelmo A, Habeck E, Poach M, Wieczorek D, Cooper K, Greenfield C, Phelan B (2014) Effects of elevated CO2 in the early life stages of summer flounder, Paralichthys dentatus, and potential consequences of ocean acidification. Biogeosciences 11:1613–1626
Checkley DM, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R (2009) Elevated CO2 enhances otolith growth in young fish. Science 324:1683
Chivers DP, McCormick MI, Nilsson GE, Munday PL, Watson S-A, Meekan MG, Mitchell MD, Corkill KC, Ferrari MCO (2014a) Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob Change Biol 20:515–522. https://doi.org/10.1111/gcb.12291
Chivers DP, Ramasamy RA, McCormick MI, Watson SA, Siebeck UE, Ferrari MCO (2014b) Temporal constraints on predation risk assessment in a changing world. Sci Total Environ 500:332–338. https://doi.org/10.1016/j.scitotenv.2014.08.059
Chung W-S, Marshall NJ, Watson S-A, Munday PL, Nilsson GE (2014) Ocean acidification slows retinal function in a damselfish through interference with GABA(A) receptors. J Exp Biol 217:323–326. https://doi.org/10.1242/jeb.092478
Clark TD, Seymour RS (2006) Cardiorespiratory physiology and swimming energetics of a high-energy-demand teleost, the yellowtail kingfish (Seriola lalandi). J Exp Biol 209:3940–3951
Clark TD, Donaldson MR, Pieperhoff S, Drenner SM, Lotto A, Cooke SJ, Hinch SG, Patterson DA, Farrell AP (2012) Physiological benefits of being small in a changing world: responses of coho salmon (Oncorhynchus kisutch) to an acute thermal challenge and a simulated capture event. PLoS One 7:e39079
Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216:2771–2782. https://doi.org/10.1242/jeb.084251
Clark TD, Roche DG, Binning SA, Speers-Roesch B, Sundin J (2017) Maximum thermal limits of coral reef damselfishes are size-dependent and resilient to near-future ocean acidification. J Exp Biol 220:3519–3526
Core Team R (2017) R: a language and environment for statistical computing R Foundation for Statistical Computing. Austria, Vienna
Cornwall CE, Hurd CL (2015) Experimental design in ocean acidification research: problems and solutions. ICES J Mar Sci 73:572–581. https://doi.org/10.1093/icesjms/fsv118
Dickson AG (1990) Standard potential of the reaction—AGCL(S) + 1/2H − 2(G) = AG(S) + HCL(AQ) and the standard acidity constant of the ion HSO4- in synthetic sea-water from 273.15-K to 318.15-K. J Chem Thermodyn 22:113–127. https://doi.org/10.1016/0021-9614(90)90074-z
Esbaugh AJ (2017) Physiological implications of ocean acidification for marine fish: emerging patterns and new insights. J Comp Physiol B 188:1–13. https://doi.org/10.1007/s00360-017-1105-6
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Faleiro F, Baptista M, Santos C, Aurélio ML, Pimentel M, Pegado MR, Paula JR, Calado R, Repolho T, Rosa R (2015) Seahorses under a changing ocean: the impact of warming and acidification on the behaviour and physiology of a poor-swimming bony-armoured fish. Conserv Physiol 3:cov009
Ferrari MCO, Munday PL, Rummer JL, McCormick MI, Corkill K, Watson S-A, Allan BJM, Meekan MG, Chivers DP (2015) Interactive effects of ocean acidification and rising sea temperatures alter predation rate and predator selectivity in reef fish communities. Glob Change Biol 21:1848–1855. https://doi.org/10.1111/gcb.12818
Flynn EE, Bjelde BE, Miller NA, Todgham AE (2015) Ocean acidification exerts negative effects during warming conditions in a developing Antarctic fish. Conserv Physiol 3:cov033
Franke A, Clemmesen C (2011) Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.). Biogeosciences 8:3697–3707. https://doi.org/10.5194/bg-8-3697-2011
Frommel AY, Maneja R, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, Piatkowski U, Reusch TBH, Clemmesen C (2012) Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat Clim Change 2:42–46. https://doi.org/10.1038/nclimate1324
Frommel AY, Schubert A, Piatkowski U, Clemmesen C (2013) Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Mar Biol 160:1825–1834. https://doi.org/10.1007/s00227-011-1876-3
Frommel AY, Maneja R, Lowe D, Pascoe CK, Geffen AJ, Folkvord A, Piatkowski U, Clemmesen C (2014) Organ damage in Atlantic herring larvae as a result of ocean acidification. Ecol Appl 24:1131–1143
Fulton TW (1904) The rate of growth of fishes. In: 22nd annual report of the fishery board of Scotland, vol 3, pp 141–241
Gagliano M, Depczynski M, Simpson SD, Moore JA (2008) Dispersal without errors: symmetrical ears tune into the right frequency for survival. Proc R Soc Lond B 275:527–534
Gamboa DA (1991) Otolith size versus weight and body-length relationships for eleven fish species of Baja California, Mexico. Fish Bull 89:701–706
Gräns A, Jutfelt F, Sandblom E, Jonsson E, Wiklander K, Seth H, Olsson C, Dupont S, Ortega-Martinez O, Einarsdottir I, Bjornsson BT, Sundell K, Axelsson M (2014) Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. J Exp Biol 217:711–717. https://doi.org/10.1242/jeb.096743
Green L, Jutfelt F (2014) Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol Lett 10:20140538. https://doi.org/10.1098/rsbl.2014.0538
Hamilton TJ, Holcombe A, Tresguerres M (2014) CO2-induced ocean acidification increases anxiety in Rockfish via alteration of GABA(A) receptor functioning. Proc R Soc Lond B 281:20132509. https://doi.org/10.1098/rspb.2013.2509
Heuer RM, Grosell M (2014) Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol 307:R1061–R1084. https://doi.org/10.1152/ajpregu.00064.2014
Heuer RM, Welch MJ, Rummer JL, Munday PL, Grosell M (2016) Altered brain ion gradients following compensation for elevated CO2 are linked to behavioural alterations in a coral reef fish. Sci Rep 6:33216
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JB, Kleypas J, Van De Leemput IA, Lough JM, Morrison TH (2017) Coral reefs in the Anthropocene. Nature 546:82
Hurst TP, Fernandez ER, Mathis JT, Miller JA, Stinson CM, Ahgeak EF (2012) Resiliency of juvenile walleye pollock to projected levels of ocean acidification. Aquat Biol 17:247–259. https://doi.org/10.3354/ab00483
Ishimatsu A, Hayashi M, Kikkawa T (2008) Fishes in high-CO2 acidified oceans. Mar Ecol Prog Ser 373:295–302. https://doi.org/10.3354/meps07823
Jutfelt F, Sundin J, Raby GD, Krång AS, Clark TD (2017) Two-current choice flumes for testing avoidance and preference in aquatic animals. Methods Ecol Evol 8:379–390
Ketcham RA (2005) Computational methods for quantitative analysis of three-dimensional features in geological specimens. Geosphere 1:32–41
Lai F, Jutfelt F, Nilsson GE (2015) Altered neurotransmitter function in CO2-exposed stickleback (Gasterosteus aculeatus): a temperate model species for ocean acidification research. Conserv Physiol 3:018. https://doi.org/10.1093/conphys/cov018
Lopes A, Morais P, Pimentel M, Rosa R, Munday P, Gonçalves E, Faria A (2016) Behavioural lateralization and shoaling cohesion of fish larvae altered under ocean acidification. Mar Biol 163:243. https://doi.org/10.1007/s00227-016-3026-4
Lueker TJ, Dickson AG, Keeling CD (2000) Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar Chem 70:105–119
Maneja RH, Frommel AY, Browman HI, Clemmesen C, Geffen AJ, Folkvord A, Piatkowski U, Durif CMF, Bjelland R, Skiftesvik AB (2013) The swimming kinematics of larval Atlantic cod, Gadus morhua L., are resilient to elevated seawater pCO2. Mar Biol 160:1963–1972
Maneja RH, Frommel AY, Browman HI, Geffen AJ, Folkvord A, Piatkowski U, Durif CMF, Bjelland R, Skiftesvik AB, Clemmesen C (2015) The swimming kinematics and foraging behavior of larval Atlantic herring (Clupea harengus L.) are unaffected by elevated pCO2. J Exp Mar Biol Ecol 466:42–48
McLeod IM, McCormick MI, Munday PL, Clark TD, Wenger AS, Brooker RM, Takahashi M, Jones GP (2015) Latitudinal variation in larval development of coral reef fishes: implications of a warming ocean. Mar Ecol Prog Ser 521:129–141
Melzner F, Goebel S, Langenbuch M, Gutowska MA, Poertner H-O, Lucassen M (2009) Swimming performance in Atlantic Cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater pCO2. Aquat Toxicol 92:30–37. https://doi.org/10.1016/j.aquatox.2008.12.011
Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL (2012) Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat Clim Change 2:858–861. https://doi.org/10.1038/nclimate1599
Milligan CL, Hooke GB, Johnson C (2000) Sustained swimming at low velocity following a bout of exhaustive exercise enhances metabolic recovery in rainbow trout. J Exp Biol 203:921–926
Möhler H, Benke D, Benson J, Luscher B, Rudolph U, Fritschy JM (1997) Diversity in structure, pharmacology, and regulation of GABAA receptors. In: Enna SJ, Bowery NG (eds) The GABA receptors. Springer Science Business Media, New York, pp 11–36
Moran D (2014) The importance of accurate CO2 dosing and measurement in ocean acidification studies. J Exp Biol 217:1827–1828
Morrongiello JR, Thresher RE, Smith DC (2012) Aquatic biochronologies and climate change. Nat Clim Change 2:849
Mu J, Jin F, Wang J, Zheng N, Cong Y (2015) Effects of CO2-driven ocean acidification on early life stages of marine medaka (Oryzias melastigma). Biogeosciences 12:3861–3868
Munday PL, Crawley NE, Nilsson GE (2009a) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388:235–242. https://doi.org/10.3354/meps08137
Munday PL, Donelson JM, Dixson DL, Endo GGK (2009b) Effects of ocean acidification on the early life history of a tropical marine fish. Proc R Soc Lond B 276:3275–3283. https://doi.org/10.1098/rspb.2009.0784
Munday PL, Gagliano M, Donelson JM, Dixson DL, Thorrold SR (2011a) Ocean acidification does not affect the early life history development of a tropical marine fish. Mar Ecol Prog Ser 423:211–221. https://doi.org/10.3354/meps08990
Munday PL, Hernaman V, Dixson DL, Thorrold SR (2011b) Effect of ocean acidification on otolith development in larvae of a tropical marine fish. Biogeosciences 8:1631–1641. https://doi.org/10.5194/bg-8-1631-2011
Munday PL, Pratchett MS, Dixson DL, Donelson JM, Endo GGK, Reynolds AD, Knuckey R (2013) Elevated CO2 affects the behavior of an ecologically and economically important coral reef fish. Mar Biol 160:2137–2144. https://doi.org/10.1007/s00227-012-2111-6
Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE (2014) Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat Clim Change 4:487–492. https://doi.org/10.1038/nclimate2195
Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sorensen C, Watson SA, Munday PL (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Change 2:201–204. https://doi.org/10.1038/nclimate1352
Norin T, Clark TD (2016) Measurement and relevance of maximum metabolic rate in fishes. J Fish Biol 88:122–151
Nowicki JP, Miller GM, Munday PL (2012) Interactive effects of elevated temperature and CO2 on foraging behavior of juvenile coral reef fish. J Exp Mar Biol Ecol 412:46–51. https://doi.org/10.1016/j.jembe.2011.10.020
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Ou M, Hamilton TJ, Eom J, Lyall EM, Gallup J, Jiang A, Lee J, Close DA, Yun S-S, Brauner CJ (2015) Responses of pink salmon to CO2-induced aquatic acidification. Nat Clim Change 5:950–955
Owens DF, Kriegstein AR (2002) Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3:715–727
Perry DM, Redman DH, Widman JC, Meseck S, King A, Pereira JJ (2015) Effect of ocean acidification on growth and otolith condition of juvenile scup, Stenotomus chrysops. Ecol Evol 5:4187–4196
Pimentel M, Pegado M, Repolho T, Rosa R (2014a) Impact of ocean acidification in the metabolism and swimming behavior of the dolphinfish (Coryphaena hippurus) early larvae. Mar Biol 161:725–729. https://doi.org/10.1007/s00227-013-2365-7
Pimentel MS, Faleiro F, Dionisio G, Repolho T, Pousao-Ferreira P, Machado J, Rosa R (2014b) Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. J Exp Biol 217:2062–2070. https://doi.org/10.1242/jeb.092635
Pistevos JC, Nagelkerken I, Rossi T, Connell SD (2017) Ocean acidification alters temperature and salinity preferences in larval fish. Oecologia 183:545
Pope EC et al (2014) European sea bass, Dicentrarchus labrax, in a changing ocean. Biogeosciences 11:2519–2530. https://doi.org/10.5194/bg-11-2519-2014
Pörtner H-O (2010) Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893
Pörtner HO, Langenbuch M, Reipschläger A (2004) Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J Oceanogr 60:705–718. https://doi.org/10.1007/s10872-004-5763-0
Rasmussen JR, Wells RM, Henty K, Clark TD, Brittain T (2009) Characterization of the hemoglobins of the Australian lungfish Neoceratodus forsteri (Krefft). Comp Biochem Physiol A Mol Integr Physiol 152:162–167
Rennie MD, Collins NC, Shuter BJ, Rajotte JW, Couture P (2005) A comparison of methods for estimating activity costs of wild fish populations: more active fish observed to grow slower. Can J Fish Aquat Sci 62:767–780
Riebesell U, Fabry VJ, Hansson L, Gattuso J-P (2010) Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union Luxembourg
Roche DG, Kruuk LE, Lanfear R, Binning SA (2015) Public data archiving in ecology and evolution: how well are we doing? PLoS Biol 13:e1002295
Rodgers R, Dalvi A (1997) Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev 21:801–810
Rodgers GG, Tenzing P, Clark TD (2016) Experimental methods in aquatic respirometry: the importance of mixing devices and accounting for background respiration. J Fish Biol 88:65-80. https://doi.org/10.1111/jfb.12848
Rosa R, Baptista M, Lopes VM, Pegado MR, Paula JR, Truebenbach K, Leal MC, Calado R, Repolho T (2014) Early-life exposure to climate change impairs tropical shark survival. Proc R Soc Lond B 281:20141738. https://doi.org/10.1098/rspb.2014.1738
Rossi T, Nagelkerken I, Simpson SD, Pistevos JCA, Watson S-A, Merillet L, Fraser P, Munday PL, Connell SD (2015) Ocean acidification boosts larval fish development but reduces the window of opportunity for successful settlement. Proc R Soc Lond B 282:20151954
Rossi T, Nagelkerken I, Pistevos JC, Connell SD (2016) Lost at sea: ocean acidification undermines larval fish orientation via altered hearing and marine soundscape modification. Biol Lett 12:20150937
Rummer JL, Stecyk JAW, Couturier CS, Watson SA, Nilsson GE, Munday PL (2013) Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv Physiol 1:cot023. https://doi.org/10.1093/conphys/cot023
Schade FM, Clemmesen C, Wegner KM (2014) Within- and transgenerational effects of ocean acidification on life history of marine three-spined stickleback (Gasterosteus aculeatus). Mar Biol 161:1667–1676
Secor DH, Dean JM, Laban EH (1992) Otolith removal and preparation for microstructural examination. In: Stevenson DK, Campana SE (eds) Otolith microstructure, examination and analysis. Canadian special publication of fisheries and aquatic sciences, Ottawa, pp 19–57
Shin LM, Liberzon I (2010) The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35:169–191
Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY (2011) Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett 7:917–920. https://doi.org/10.1098/rsbl.2011.0293
Stewart A, Wu N, Cachat J, Hart P, Gaikwad S, Wong K, Utterback E, Gilder T, Kyzar E, Newman A (2011) Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog Neuro-Psychopharmacol Biol Psychiatry 35:1421–1431
Sundin J, Jutfelt F (2016) 9–28 d of exposure to elevated pCO2 reduces avoidance of predator odour but had no effect on behavioural lateralization or swimming activity in a temperate wrasse (Ctenolabrus rupestris). ICES J Mar Sci 73:620–632
Sundin J, Amcoff M, Mateos-González F, Raby GD, Jutfelt F, Clark TD (2017) Long-term exposure to elevated carbon dioxide does not alter activity levels of a coral reef fish in response to predator chemical cues. Behav Ecol Sociobiol 71:108
Tix JA, Hasler CT, Sullivan C, Jeffrey JD, Suski CD (2017) Elevated carbon dioxide has the potential to impact alarm cue responses in some freshwater fishes. Aquat Ecol 51:59. https://doi.org/10.1007/s10452-016-9598-8
Tresguerres M, Hamilton TJ (2017) Acid–base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J Exp Biol 220:2136–2148
Weissgerber TL, Milic NM, Winham SJ, Garovic VD (2015) Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol 13:e1002128
Welch MJ, Watson S-A, Welsh JQ, McCormick MI, Munday PL (2014) Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat Clim Change 4:1086–1089. https://doi.org/10.1038/nclimate2400
Acknowledgements
This work was funded by the Swedish Research Council Formas (2013-947, JS), the Swedish Research Council VR (637-2014-449, MA), the Australian Research Council (Future Fellowship FT180100154, TDC), the Wenner-Gren Foundation (JS, MA), the Society for Experimental Biology travel grant and the Company of Biologists Travel Grant (JEBTF-150422, JS), the Fulbright Foundation (MA), Inez Johanssons stiftelse (FMG), and through an Australian Endeavour Research Fellowship (GDR). We thank F. Jutfelt for contributing vital equipment, J. Maisano for assistance with the CT-scan, and A. Severati and C. Schlott for collecting wild fish. Invaluable statistical support was provided by E. Hale and C. Blanco, statistical consultants at the University of Texas at Austin. Further support and facilities were provided by the Australian Institute of Marine Science (AIMS). Special thanks to the SeaSim staff at AIMS for logistical support.
Author information
Authors and Affiliations
Contributions
All authors designed and performed the experiments. MA, JS and TDC analysed the data. JS and MA wrote the manuscript with significant contributions in addition to approval from all other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing or financial interests.
Additional information
Communicated by Donovan P German.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sundin, J., Amcoff, M., Mateos-González, F. et al. Long-term acclimation to near-future ocean acidification has negligible effects on energetic attributes in a juvenile coral reef fish. Oecologia 190, 689–702 (2019). https://doi.org/10.1007/s00442-019-04430-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04430-z