Abstract
Resource availability can significantly alter host–parasite dynamics. Abundant food can provide more resources for hosts to resist infections, but also increase host tolerance of infections by reducing competition between hosts and parasites for food. Whether abundant food favors host resistance or tolerance (or both) might depend on the type of resource that the parasite exploits (e.g., host tissue vs. food), which can vary based on the stage of infection. In our study, we evaluated how low and high resource diets affect Cuban tree frog (Osteopilus septentrionalis) resistance and tolerance of a skin-penetrating, gut nematode Aplectana sp. at each stage of the infection. Compared to a low resource diet, a high resource diet enhanced frog resistance to worm penetration and tolerance while worms traveled to the gut. In contrast, a low resource diet increased resistance to establishment of the infection. After the infection established and worms could access food resources in the gut, a high resource diet enhanced host tolerance of parasites. On a high resource diet, parasitized frogs consumed significantly more food than non-parasitized frogs; when food was then restricted, mass of non-parasitized frogs did not change, whereas mass of parasitized frogs decreased significantly. Thus, a high resource diet increased frog tolerance of established worms because frogs could fully compensate for energy lost to the parasites. Our study shows that host–parasite dynamics are influenced by the effect of resource availability on host resistance and tolerance, which depends on when parasites have access to food and the stage of infection.
Similar content being viewed by others
Introduction
Hosts can reduce parasite damage by decreasing parasite fitness (resistance) or reducing the harm that an infection causes (virulence) without negatively affecting parasite fitness (tolerance) (Miller et al. 2006; Råberg et al. 2007; Read et al. 2008; Medzhitov et al. 2012; Sears et al. 2013). Ecological studies have found that host resistance and tolerance can act either together or alone in response to parasitism (Sternberg et al. 2012; Sorci 2013; Sears et al. 2015; Knutie et al. 2016), suggesting that the relationship between these two defenses may be context dependent. The context in which hosts favor either or both defenses might depend on environmental factors, such as resource availability (McKenzie and Townsend 2007; Johnson et al. 2010; Becker et al. 2015). For example, low resource availability can lead to less food for the parasite (Kyriazakis et al. 1998), but also to poor overall health for the host, which might alter physiological and behavioral mechanisms related to host defenses against parasites (Cotter et al. 2011).
Resistance mechanisms, such as immune responses, require energetic investment by the host (Read et al. 2008; Rohr et al. 2010) and are therefore resource dependent. For example, hosts on a supplemented diet can be more resistant to their parasites than hosts on an unsupplemented diet (Cornet et al. 2014), presumably because they have more energy to invest in resistance mechanisms. Studies have focused on interactions between nutritional status and immunological resistance because immune function can be condition-dependent (reviewed by Sheldon and Verhulst 1996; Lochmiller and Deerenberg 2000); these studies demonstrate that immune responses can be energetically costly to produce and only hosts in good condition may be physiologically able to invest extensively in these defenses (Svensson et al. 1998). However, resistance mechanisms, such as inflammation, may come at a cost because they can cause collateral damage to hosts (Sears et al. 2011; Cornet et al. 2014).
Host tolerance of parasitism can also depend on resource availability. Tolerance can be illustrated by the slope of the reaction norm between host fitness and parasite burden (Simms 2000); higher host tolerance is indicated with a shallower slope, whereas lower tolerance has a steeper negative slope. An example of condition-dependent host tolerance is when avian parents from parasite-infested nests feed their offspring more than parents from non-parasitized nests (“parental compensation hypothesis”; Christe et al. 1996; Tripet and Richner 1997; Knutie et al. 2016). Consequently, tolerant offspring do not suffer a cost of parasitism (regardless of burden) because parents are able to help their offspring fully compensate for energy lost to parasitism. The extensive literature on plant tolerance of herbivory may also help explain when host tolerance of parasites is favored. Such studies suggest that the effect of resource availability on host tolerance depends on which factors (e.g., water, nutrients, light) limit plant fitness and where on the plant herbivory occurs (Wise and Abrahamson 2005, 2007). For example, when plant fitness is most limited by water and herbivores exploit the xylem in the roots, environments with higher precipitation increase plant tolerance to herbivory more than those with low precipitation. In contrast, an environment with high precipitation does not enhance tolerance to herbivory when herbivores exploit alternative tissues that are less important in water regulation, such as leaves.
The effect of resource availability on resistance and tolerance of parasites by animal hosts might also depend on whether the parasites are feeding on the resources consumed by the host or on host tissue itself. For many vertebrate host species, higher food availability decreases abundance of parasites that consume host tissue (Cressler et al. 2014), which suggests that these hosts are investing extra resources to resist their parasites and associated tissue damage. In contrast, increased food availability for other animal hosts, such as Daphnia, favors increased tolerance of parasites with whom they share food with, likely because more food for both the host and parasite reduces competition (Vale et al. 2011). The type of resource the parasites are consuming and/or damaging can also depend on the stage of the infection, and thus likely influences different defense strategies (Howick and Lazzaro 2014). Together, these studies suggest that the type of resource exploited by the parasite, which likely depends on the stage of infection, might determine how resource availability influences animal host tolerance and resistance of parasitism.
The goal of our study was to determine the effect of food resource availability on host resistance and tolerance of parasites. We studied a parasitic nematode Aplectana sp. that infects Cuban tree frogs (Osteopilus septentrionalis) (Ortega et al. 2015). Aplectana sp. has a direct life cycle: juvenile larvae penetrate frog skin and then, in approximately 3 weeks, establish, mature and reproduce in the gastrointestinal (GI) tract. Worm eggs and larvae (they are ovoviviparous) are defecated by frogs, and after approximately a week of development, juveniles can infect the next host. The advantages of this system for addressing the role of resources on infection and disease are manifold. First, direct life cycles make experimental manipulations of parasites in the host more straightforward than parasites with complex (indirect) life cycles. Second, the free-living stage of this parasite does not have access to food consumed by the host when attempting to penetrate host skin but does have access after entering the GI tract of a host; this allows us to assess host resistance and tolerance at different stages of the infection when parasites have and do not have access to food resources consumed by the host.
In our study, we conduct two experiments to determine the effect of a high and low resource diet on Cuban tree frog resistance and tolerance to Aplectana sp. at each stage of infection: initial penetration, establishment, and post-establishment. In our first experiment, we determine how food levels affect host resistance to Aplectana sp. during the initial skin penetration stage and host resistance and tolerance while the infection is establishing. In our second experiment, we determine how changes in food levels affect host tolerance of parasitism after the infection has established in the gut. We predict that resource availability affects both host resistance and tolerance to parasitism but that this effect depends on the stage of infection and whether parasites and/or hosts have access to resources. While penetrating the host and establishing in the gut, parasites do not have access to food resources in the gut; thus, we predict that a high resource diet will increase host resistance because hosts have extra resources to allocate to immunological defenses (Fig. 1a). After the worm establishes in the gut and has access to food resources there, we predict that a high resource diet favors host tolerance of worms more so than a low resource diet (Fig. 1b).
Predictions for the effect of resource availability on the relationship between host health and parasite abundance. a When only hosts have access to food resources, hosts with high resources (solid line) will have fewer parasites than hosts with low resources (dotted line). b Once the infection has established, hosts with high resources will be more tolerant of effects of parasites compared to hosts with low resources (figure modified from Råberg et al. 2007)
Materials and methods
Cuban tree frog tadpoles were collected from the University of South Florida (USF) Botanical Gardens and brought into captivity at the USF animal facilities. Tadpoles were maintained in pond water until they reached metamorphosis and then were placed in individual cups (6 cm high × 12 cm diameter) with moist Sphagnum sp. moss. All juvenile frogs were maintained in the laboratory (12 h light–dark cycle, 22 °C) and fed crickets (ad libitum) until experiments started, which occurred approximately 2 months after metamorphosis. All crickets were lightly and evenly dusted with a pinch of Rep-cal® multivitamin and calcium powder before they were given to the frogs.
For the first experiment, we tested the effect of resources on host resistance and tolerance against worm penetration and establishment. We randomly assigned 30 frogs to either a high (n = 15) or low resource diet (n = 15). We first determined that non-parasitized frogs ate a mean ± SE of 5.90 ± 0.29 crickets, twice per week. Before the experimental worm infection, frogs on a high resource diet were provided with eight crickets and frogs on a low resource diet were provided with three crickets (50% of average consumption), twice per week. After 3 weeks on the feeding regime, frogs were exposed to 25 infectious juvenile worms (collected from naturally parasitized adults). To infect frogs, individuals were placed in petri dishes (100 mm diameter) with air holes on the lid top. Thus, frogs had little space to move and avoid parasites. The top and bottom of a petri dish were sealed together with parafilm. Frogs were exposed to worms by pipetting the worms suspended in 3 mL of autoclaved pond water through the lid holes. After 24 h of exposure in petri dishes, frogs were returned to their individual cups with Sphagnum moss. The worms remaining in the dish were then counted under a microscope to determine the number of worms that penetrated each frog. Frogs remained on either a high or low resource diet for three additional weeks. Frog survival was recorded daily.
Three weeks after exposure to Aplectana worms, frogs were euthanized and a blood sample was collected for the antibody-mediated immune assay. Frogs were also necropsied to count the number of worms in their GI tract. Three weeks after worm exposure is the estimated amount of time it takes for worms to establish in the GI tract and begin consuming resources from the gut of frogs, and is when we detect a peak antibody-mediated immune response in the frogs (S. Knutie personal observation).
Frogs were weighed to the nearest thousandth of a gram (g) on the day that the frogs: (1) began their diet treatment, (2) were infected, and (3) were euthanized. Host tolerance can be quantified as the reaction norm between parasite abundance and host fitness (e.g., survival) but many parasites have sublethal, rather than lethal, effects on their host. Therefore, we quantified host tolerance as the reaction norm between parasite abundance and host body mass. We acknowledge that body mass of captive adult animals may not be equivalent to the body mass of the same animals in the wild; however, we used body mass as a metric of host health because the frogs in our experiment were still growing (<2 months post-metamorphosis), and thus healthy frogs should be increasing in body mass.
We also determined whether frogs mounted an antibody-mediated immune response to Aplectana sp. in a supplemental experiment. Briefly, frogs (<2 months post-metamorphosis) were either exposed to 20 juvenile worms (n = 5), using the same method as the previous experiment, or sham-exposed where we pipetted the same solution without worms into petri dishes (n = 10). Three weeks after frogs were exposed to worms, frogs were euthanized and blood was collected to quantify antibody levels.
Enzyme-linked immunosorbent assays (ELISA) were used to detect the presence of IgY antibodies in frog plasma. Each well of a ninety-six well plates were coated with 100 μL of individual frog serum diluted 1:100 in carbonate coating buffer (0.05 M, pH 9.60). Each sample was applied in triplicate on the plates. Plates were incubated overnight at 4 °C. They were then washed and coated with 200 μL/well of bovine serum albumin (BSA) blocking buffer and incubated for 30 min at room temperature on an orbital table. Between each of the following steps, plates were washed five times with a Tris-buffered saline wash solution, loaded as described, and incubated for 1 h on an orbital table at room temperature. Plates were then loaded with 100 μL/well of primary detection antibody (Goat-αAlligator-IgG, diluted 1:1000; Bethyl) for 1 h, washed, then loaded with 100 μL/well of a conjugate detection antibody (Rabbit-αGoat-IgG, diluted 1:5000; Bethyl) for 1 h. Finally, plates were loaded with 100 μL/well of peroxidase substrate (tetramethylbenzidine, TMB: Bethyl Laboratories) and incubated for exactly 30 min. The reaction was halted using 100 μL/well of stop solution (Bethyl Laboratories). Optical density (OD) was measured with a spectrophotometer (BioTek, PowerWave HT, 450-nanometer filter).
We conducted a second experiment to explore the effect of resources on frog tolerance of parasitism after the infection had established. We randomly assigned 30 Cuban tree frogs to either a parasitized (n = 15) or non-parasitized treatment (n = 15). Frogs from the parasitized treatment received 20 juvenile worms, whereas frogs from the non-parasitized treatment received a sham-treatment where we pipetted the same solution without worms into petri dishes. Remaining worms were added to the frogs’ cups and 1 week later, 20 additional juvenile worms were added to cups for a total of 40 worms. We increased the number of worms that the frogs were exposed to in this experiment (compared to 25 worms in the first experiment) to increase the likelihood that frogs would become infected. Therefore, we acknowledge that infection intensity could differ between experiments. After worm exposure, frogs were fed a high resource diet of vitamin- and mineral-dusted crickets. Once per week, bedding was changed and frogs were weighed. We checked frog survival daily.
Four weeks after the initial exposure, we collected and weighed feces from their cups to determine whether the infection was successful. We then determined the mean number of crickets eaten by parasitized and non-parasitized frogs by giving each frog four crickets and counting the number remaining in the cup after 2 days. Over the following 4 weeks, we determined the effect of food restriction on the mass of frogs with low or high resource feeding trials (two trials each). During two of the trials (6 and 8 weeks after worm exposure), all frogs were fed an average number of crickets eaten by non-parasitized frogs (two crickets) and weighed 4 days later with the expectation that when fed only two crickets, parasitized frogs would lose more weight than non-parasitized frogs. During the other two trials (7 and 9 weeks after worm exposure), all frogs were fed a high resource diet (four crickets) and weighed 4 days later with the expectation that parasitized frogs would not lose more weight than non-parasitized frogs. When a frog died before the end of the experiment, we extracted the GI tract and counted the number of adult worms. After the fourth feeding trial, frogs were euthanized, necropsied, and the number of adult worms in each frog was quantified.
Statistical analyses
All statistical analyses were conducted in RStudio (2013, version 0.98.1062) and graphs were made in Prism (2008, version 5b). For the first experiment, we compared proportional change in mass and antibody levels between low and high resource diet frogs using general linear models (GLM) with Gaussian errors. We also used GLMs with binomial errors to determine the effect of treatment on: (1) proportion of worms that penetrated frogs out of the number to which they were exposed and (2) proportion of worms that established in the gut out of the number of worms that penetrated. These analyses were conducted using the glm function. We initially used body mass as a covariate for all models but it was excluded from all models because it did not account for a significant amount of variation.
For the second experiment, we quantified the effect of parasitism on the proportion of frogs with feces and the proportion of crickets eaten (in two feeding trials) using general linear mixed models (GLMM) with binomial errors and treating individual frogs as a random effect. We determined the effect of treatment on frog mass using a GLMM with Gaussian errors by treating trial, parasitism, feeding regime, and the interaction between parasitism and feeding regime as fixed effects and individual frog as a random effect. Gaussian and binomial analyses were conducted using the lmer and glmer functions, respectively. We determined the effect of worm treatment on survival using a censored Cox proportional hazard model with the coxph function. Probability values were calculated using log-likelihood ratio tests using the Anova function in the car package.
Results
For the first experiment, all frogs survived the entire 6-week experiment. Frogs, on average, gained 24.4% more mass on a high resource diet than on a low resource diet (GLM, χ 2 = 18.03, df = 1, P < 0.0001). When considering mass before and during infection separately, frogs on a high resource diet gained more mass both before infection (GLM, χ 2 = 13.15, df = 1, P < 0.001) and while the infection was establishing (GLM, χ 2 = 4.91, df = 1, P = 0.03) compared to frogs on a low resource diet.
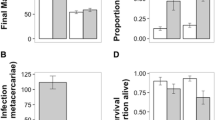
Frogs on a low resource diet were penetrated by 35% more worms than frogs on a high resource diet (GLM, χ 2 = 3.80, df = 1, P = 0.05; Fig. 2a). Proportional change in mass (from the start of the experiment to parasite exposure) negatively predicted the number of worms that penetrated successfully (GLM, χ 2 = 3.52, df = 1, P = 0.05; Fig. 2b). The number of worms that penetrated successfully affected the mass of frogs while the infection was establishing, but this relationship depended on diet treatment (GLM, χ 2 = 6.89, df = 1, P = 0.009). On a high resource diet, frogs did not lose mass with increasing parasite abundance (number of worms penetrated), whereas on a low resource diet, frog mass significantly decreased with increasing parasite abundance. Three weeks after worm exposure, only 30% of worms that penetrated frogs on a low resource diet established in their GI tracts, whereas 62% of worms that penetrated frogs on a high resource diet established successfully (GLM, χ 2 = 17.14, df = 1, P < 0.0001; Fig. 2c). These opposing effects of diet treatments on proportion of worms that penetrated versus proportion that established in the GI tract resulted in no significant difference in the total number of worms in the GI tract between diet treatments (GLM, χ 2 = 0.63, df = 1, P = 0.43; Fig. 2d).
Effect of resources on host resistance against initial worm penetration and establishment in the gut. a Low resource diet frogs were penetrated by proportionally more worms compared to high resource diet frogs (n = 15 for each treatment); b proportional change in mass was negatively related to the proportion of worms that penetrated the frogs; c high resource diet frogs had proportionally more worms establish in frog guts (out of the number that penetrated) compared to low resource diet frogs; d total number of worms that established in the gut did not differ significantly between treatments. Each panel displays means (±1 SE)
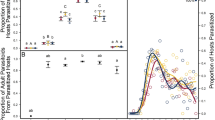
Infected frogs mounted a significantly higher antibody response to Aplectana compared to uninfected frogs (GLM, χ 2 = 16.02, df = 1, P < 0.0001). The number of worms that established in the gut was positively related to antibody levels (GLM, χ 2 = 6.46, df = 1, P = 0.01; Fig. 3a). When we controlled for number of worms in the gut, we found a significant effect of treatment on antibody levels (GLM, χ 2 = 4.57, df = 1, P = 0.03; Fig. 3b) with frogs on a low resource diet producing a higher antibody-mediate response compared to hosts on a high resource diet.
Relationship between antibody-mediated immune response and parasitism across diet treatments. a Parasite abundance was positively related to antibody levels for low (dotted line) and high (solid line) resource diet; b high resource diet frogs had lower antibody levels [optical density (OD) values] compared to low resource diet frogs (low resource diet: n = 14 frogs, high resource diet: n = 15 frogs). Means are displayed with ±1 SE
For the second experiment, all frogs intentionally exposed to larval worms were infected with at least three adult worms in the gut (mean ± SE = 10.46 ± 1.14 worms, range 3–15); hereafter, these frogs are referred to as parasitized frogs. We found no evidence of infections in our control frogs, and thus hereafter, they are referred to as non-parasitized frogs. Parasitism did not significantly affect survival (non-parasitized: 12/15 survived; parasitized: 9/15 survived; Cox proportional hazard, χ 2 = 1.31, df = 1, P = 0.25), and only frogs that lived for at least 1 week after worm or sham exposures were included in our mass change analyses.
Parasitized frogs defecated more frequently than non-parasitized frogs (GLM, χ 2 = 6.17, df = 1, P = 0.01; Fig. 4a), but the mass of any individual bowel movement did not differ significantly between parasitized and non-parasitized groups (mean ± SE: 0.02 ± 0.004, 0.02 ± 0.01 g, respectively; GLM, χ 2 = 0.71, df = 1, P = 0.40). Across two feeding trials, parasitized frogs consumed 52% more crickets than non-parasitized frogs (GLMM, parasitism: χ 2 = 7.95, df = 1, P = 0.005, trial: χ 2 = 0.40, df = 1, P = 0.53, interaction: χ 2 = 0.01, df = 1, P = 0.91; Fig. 4b). This increased feeding rate resulted in an interaction between parasitism and diet on proportional mass change (GLMM, χ 2 = 3.94, df = 1, P = 0.05). During resource manipulation trials, both parasitized and non-parasitized frogs gained mass on a high resource diet but parasitized frogs on a low resource diet lost mass (Fig. 4c). Similar results were obtained when considering host tolerance of worm infections. On a high resource diet, frogs did not lose mass in response to increasing parasite abundance, whereas on a low resource diet, frog mass significantly decreased with increasing parasite loads (GLMM, χ 2 = 4.82, df = 1, P = 0.03; Fig. 4d).
Effect of resources on host tolerance of parasitism after infection had established. a Parasitized frogs defecated significantly more often (n = 11) than non-parasitized frogs (n = 13); b parasitized frogs (n = 11) ate significantly more crickets than non-parasitized frogs (n = 13) after 2 days and across two trials; c mean (±SE) proportional change in frog mass between weeks during high access to food or from high to low access to food (parasitized, trial 1: n = 11 frogs, trial 2: n = 9; non-parasitized, trial 1: n = 13, trial 2: n = 12). A value of zero indicates no change in mass, while a positive value indicates an increase and a negative value indicates a decrease in mass. Non-parasitized and parasitized frogs that were provided a high resource diet and non-parasitized frogs provided a low resource diet gained mass; in contrast, parasitized frogs on a low resource diet lost mass; d host tolerance of Aplectana worms on high and low resource diets. While on a high resource diet (black points and line), mass did not significantly vary with parasite abundance. In contrast, mass decreased with increasing parasite abundance on a low resource diet (gray points and line) in the same frogs
Discussion
Resource availability affected both host resistance and tolerance of parasitism. During the skin penetration stage when only hosts had access to resources, we showed that high resource availability enhanced host resistance (Fig. 2a). While worms were travelling to the gut, a high resource diet favored tolerance of the establishing infection (by allowing hosts to maintain mass despite an increasing parasite load), whereas a low resource diet reduced host tolerance. Frogs on a low resource diet resisted worm establishment in the gut more so than frogs on a high resource diet (Fig. 2c). Furthermore, low resource diet frogs produced a higher antibody-mediated immune response while the infection established (Fig. 3b), which was positively related to parasite abundance (Fig. 3a). After the infection had established, high resources caused a net increase in host tolerance of infections (shallower slope between parasite abundance and mass loss; Fig. 4c, d). Frogs on a high resource diet ate and defecated more when parasitized than not parasitized (Fig. 4a, b), suggesting that frogs increased their nutrient intake to recover energy lost from infection. This, in turn, allowed parasitized frogs on a high resource diet to maintain a similar growth rate as non-parasitized frogs. Overall, we were able to show that high resource availability increases resistance to becoming infected and tolerance once infected but at the cost of reduced resistance to parasite establishment.
A low resource diet favored host resistance to worm establishment in the gut, which contradicted our prediction. Kyriazakis et al. (1998) suggested that a low resource diet could increase host resistance to infection by starving the parasite, but may also favor host immunological resistance. For example, increased glucose intake by domesticated animal hosts can decrease immunological resistance to gastrointestinal nematodes (Bown et al. 1991). Interestingly, we found that parasitized frogs on a low resource diet produced a larger antibody-mediated immune response compared to parasitized frogs on a high resource diet. However, we found a positive relationship between antibody levels and the number of worms in the gut, which suggests that frogs have acquired immunity to the parasite; that is, during the first exposure to parasites, the antibody-mediated response is mounted, but is not necessarily effective at reducing parasite fitness, and then during subsequent parasite exposures, hosts are immunologically primed against the parasite and can more effectively reduce parasite fitness. We did not quantify the effect of resources on acquired immunity during subsequent parasite exposure, so this idea requires further investigation, but our results suggest two interesting ideas: (1) the cost of a more local antibody-mediated immune response in the gut (compared to the potentially costly systemic resistance in the skin) does not outweigh the future cost of parasitism on a low resource diet, and (2) that frogs on a high resource diet are not as primed against future infections compared to frogs on a low resource diet.
A high resource diet favored host resistance to skin penetration by the parasite as well as host tolerance while parasites traveled to the gut. These results suggest that either the cost of resistance during worm penetration has a lasting effect on frog health or that frogs suffer an additional energetic cost while worms travel to the gut. We did not quantify the resistance mechanism at the skin penetration stage, but frogs likely invested in an inflammatory response to prevent penetration of the worms. Inflammation can be costly by causing collateral damage to the host (Sears et al. 2011; Adelman et al. 2013). Therefore, frogs on a high resource diet might be better able to repair collateral damage or recover energy lost during the skin penetration stage compared to the frogs with fewer resources. A future study could determine whether frogs invest in an inflammatory response to worms by quantifying epidermal cell number and immigration of leukocytes on the skin (Owen et al. 2009) and then test whether resource availability mediates the effect of the inflammatory response on host health while the infection establishes.
A high resource diet also favored host tolerance after the infection established in the gut and worms had access to food resources. Other studies have also found that higher resource availability can increase host tolerance of parasites (Sternberg et al. 2012; Howick and Lazzaro 2014). For example, Vale et al. (2011) found that on a high resource diet, competition for food resources between Daphnia hosts and their bacterial parasites were reduced allowing for increased host tolerance. Additionally, baseline metabolic rate of hosts can increase in responses to parasitism (Connors and Nickol 1991; Careau et al. 2010) and in turn, hosts may increase food intake to compensate for energy lost to parasitism (i.e., ‘resource compensation hypothesis’; Christe et al. 1996; Tripet and Richner 1997; Murray et al. 1998). However, resource compensation likely comes with a cost to hosts. For example, increased foraging activity can result in higher predation rates, lower investment in reproductive success, and higher parasite exposure (Anholt and Werner 1995; Ezenwa 2004). With all the potential costs of increased foraging activity, why do hosts increase foraging after the infection has established instead of decreasing foraging efforts to starve (i.e., resist) the parasite? It is likely because the cost of losing mass outweighs the cost of attempting to clear the parasites, but this hypothesis was not explored in our study.
Host tolerance against parasites likely influences disease dynamics. When resources are abundant, our results suggest that host tolerance to parasitism increases more so than resistance. Therefore, virulence might be relatively low, which could allow for parasites to persist in high numbers (Beldomenico and Begon 2010). As resources are depleted, the effects of parasitism are predicted to intensify, increasing parasite-induced mortality of hosts and resistance of surviving hosts to new infections, and thus decreasing parasite abundance. Resource availability did not affect the total number of worms that established in the gut. However, resources may affect reproductive success or biomass (i.e., measure of virulence for macroparasites) of parasites, and thus over time, parasite numbers may increase as host tolerance increases in the population (Vale et al. 2011). We did not quantify fecundity or biomass of worms in frogs under varying resource conditions but this question could be addressed in the future.
The net effect of resources on resistance was likely zero given that the number of worms that established in the gut did not differ between diet treatments. Thus, overall, increased resources primarily affected host tolerance by allowing frogs to maintain mass despite increasing parasite loads. Previous studies found that when resources are abundant, vertebrate hosts generally had fewer microparasites (reviewed by Cressler et al. 2014), which suggests that hosts invest extra resources in resistance against microparasites. Higher resource availability can increase microparasite virulence while in a host so hosts might invest more in resistance mechanisms to reduce parasite fitness. However, when the cost of parasitism is less than the cost of resistance, hosts may alternatively invest in tolerance mechanisms (Vale et al. 2011). Higher resource availability can increase the virulence of macroparasites by increasing their biomass but may not increase virulence to the extent that resources affect microparasite virulence because macroparasite offspring are typically released from a host to infect a different host. Thus, there might be less selective pressure for hosts to evolve resistance of macroparasites once they have established. Instead, our results suggest that hosts invest extra resources into tolerance mechanisms (e.g., via energy compensation; Tripet and Richner 1997; Vale et al. 2011; Knutie et al. 2016) to minimize the costs of their current infections without potentially engaging in a detrimental arms race with their parasite.
Our study is among the first to experimentally demonstrate that resources affect host tolerance and resistance of parasites during different stages of the infection. These results highlight the value of partitioning the effects of resource amendments on the host and parasite to: (1) better understand the direct effects of resources on each species and the net effect of resources on the interaction, and (2) reveal that resource availability differentially affects host defenses at different stages of the infection process. Resource availability often fluctuates in dynamic environments, including supplemental feeding of wildlife by humans, and likely plays a significant role in altering host–parasite interactions (Becker et al. 2015). Therefore, understanding the complexities of host–parasite interactions in other systems as well as how to manage disease should involve studying host defenses during different stages of the infection while manipulating or controlling resource availability.
References
Adelman JS, Kirkpatrick L, Grodio JL, Hawley DM (2013) House finch populations differ in early inflammatory signaling and pathogen tolerance at the peak of Mycoplasma gallisepticum infection. Am Nat 181:674–689. doi:10.1086/670024
Anholt BR, Werner EE (1995) Interaction between food availability and predation mortality mediated by adaptive behavior. Ecology 76:2230–2234. doi:10.2307/1941696
Becker DJ, Streicker DG, Altizer S (2015) Linking anthropogenic resources to wildlife-pathogen dynamics: a review and meta-analysis. Ecol Lett 18:483–495. doi:10.1111/ele.12428
Beldomenico PM, Begon M (2010) Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol Evol. doi:10.1016/j.tree.2009.06.015
Bown MD, Poppi DP, Sykes AR (1991) The effect of post-ruminal infusion of protein or energy on the pathophysiology of Trichostrongylus colubriformis infection and body composition in lambs. Aust J Agric Res 42:253–267. doi:10.1071/AR9910253
Careau V, Thomas DW, Humphries MM (2010) Energetic cost of bot fly parasitism in free-ranging eastern chipmunks. Oecologia 162:303–312. doi:10.1007/s00442-009-1466-y
Christe P, Richner H, Oppliger A (1996) Begging, food provisioning, and nestling competition in great tit broods infested with ectoparasites. Behav Ecol 7:127–131. doi:10.1093/beheco/7.2.127
Connors VA, Nickol BB (1991) Effects of Plagiorhynchus cylindraceus (Acanthocephala) on the energy metabolism of adult starlings, Sturnus vulgaris. Parasitology 103:395–402. doi:10.1017/S0031182000059916
Cornet S, Bichet C, Larcombe S et al (2014) Impact of host nutritional status on infection dynamics and parasite virulence in a bird-malaria system. J Anim Ecol 83:256–265. doi:10.1111/1365-2656.12113
Cotter SC, Simpson SJ, Raubenheimer D, Wilson K (2011) Macronutrient balance mediates trade-offs between immune function and life history traits. Funct Ecol 25:186–198. doi:10.1111/j.1365-2435.2010.01766.x
Cressler CE, Nelson WA, Day T, McCauley E (2014) Disentangling the interaction among host resources, the immune system and pathogens. Ecol Lett 17:284–293. doi:10.1111/ele.12229
Ezenwa VO (2004) Selective defecation and selective foraging: antiparasite behavior in wild ungulates? Ethology 110:851–862. doi:10.1111/j.1439-0310.2004.01013.x
Howick VM, Lazzaro BP (2014) Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol Biol 14:56. doi:10.1186/1471-2148-14-56
Johnson PTJ, Townsend AR, Cleveland CC et al (2010) Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecol Appl 20:16–29
Knutie SA, Owen JP, McNew SM et al (2016) Galápagos mockingbirds tolerate introduced parasites that affect Darwin’s finches. Ecology 97:940–950. doi:10.1890/15-0119.1
Kyriazakis I, Tolkamp B, Hutchings M (1998) Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim Behav 56:265–274. doi:10.1006/anbe.1998.0761
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98. doi:10.1034/j.1600-0706.2000.880110.x
McKenzie VJ, Townsend AR (2007) Parasitic and infectious disease responses to changing global nutrient cycles. EcoHealth 4:384–396. doi:10.1007/s10393-007-0131-3
Medzhitov R, Schneider D, Soares M (2012) Disease tolerance as a defense strategy. Science 335:936–941. doi:10.1126/science.1214935
Miller MR, White A, Boots M (2006) The evolution of parasites in response to tolerance in their hosts: the good, the bad, and apparent commensalism. Evolution 60:945–956. doi:10.1111/j.0014-3820.2006.tb01173.x
Murray DL, Keith LB, Cary JR (1998) Do parasitism and nutritional status interact to affect production in snowshoe hares? Ecology 79:1209–1222. doi:10.2307/176737
Ortega N, Price W, Campbell T, Rohr JR (2015) Acquired and introduced macroparasites of the invasive Cuban tree frog, Osteopilus septentrionalis. Int J Parasitol Parasites Wildl 4:379–384. doi:10.1016/j.ijppaw.2015.10.002
Owen JP, Delany ME, Cardona CJ et al (2009) Host inflammatory response governs fitness in an avian ectoparasite, the northern fowl mite (Ornithonyssus sylviarum). Int J Parasitol 39:789–799. doi:10.1016/j.ijpara.2008.12.008
Råberg L, Sim D, Read AF (2007) Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318:812–814. doi:10.1126/science.1148526
Read AF, Graham AL, Råberg L (2008) Animal defenses against infectious agents: is damage control more important than pathogen control. PLoS Biol 6:2638–2641. doi:10.1371/journal.pbio.1000004
Rohr JR, Raffel TR, Hall CA (2010) Developmental variation in resistance and tolerance in a multi-host–parasite system. Funct Ecol 24:1110–1121. doi:10.1111/j.1365-2435.2010.01709.x
Sears BF, Rohr JR, Allen JE, Martin LB (2011) The economy of inflammation: when is less more? Trends Parasitol 27:382–387. doi:10.1016/j.pt.2011.05.004
Sears BF, Snyder PW, Rohr JR (2013) Infection deflection: hosts control parasite location with behaviour to improve tolerance. Proc R Soc Lond B 280:20130759. doi:10.1098/rspb.2013.0759
Sears BF, Snyder PW, Rohr JR (2015) Host life history and host–parasite syntopy predict behavioural resistance and tolerance of parasites. J Anim Ecol 84:625–636. doi:10.1111/1365-2656.12333
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321. doi:10.1016/0169-5347(96)10039-2
Simms EL (2000) Defining tolerance as a norm of reaction. Evol Ecol 14:563–570. doi:10.1023/A:1010956716539
Sorci G (2013) Immunity, resistance and tolerance in bird–parasite interactions. Parasite Immunol 35:350–361. doi:10.1111/pim.12047
Sternberg ED, Lefèvre T, Li J et al (2012) Food plant derived disease tolerance and resistance in a natural butterfly-plant-parasite interactions. Evolution 66:3367–3376. doi:10.1111/j.1558-5646.2012.01693.x
Svensson E, Råberg L, Koch C, Hasselquist D (1998) Energetic stress, immunosuppression and the costs of an antibody response. Funct Ecol 12:912–919. doi:10.1046/j.1365-2435.1998.00271.x
Tripet F, Richner H (1997) Host responses to ectoparasites: food compensation by parent blue tits. Oikos 78:557–561. doi:10.2307/3545617
Vale PF, Wilson AJ, Best A et al (2011) Epidemiological, evolutionary, and coevolutionary implications of context-dependent parasitism. Am Nat 177:510–521. doi:10.1086/659002
Wise MJ, Abrahamson WG (2005) Beyond the compensatory continuum: environmental resource levels and plant tolerance of herbivory. Oikos 109:417–428. doi:10.1111/j.0030-1299.2005.13878.x
Wise MJ, Abrahamson WG (2007) Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. Am Nat 169:443–454. doi:10.1086/512044
Acknowledgements
We thank Jeb Owen for advice on the immunoassay protocol. This research was supported by Grants from the National Science Foundation (EF-1241889), National Institutes of Health (R01GM109499, R01TW010286), US Department of Agriculture (NRI 2006-01370, 2009-35102-0543), and US Environmental Protection Agency (CAREER 83518801) to JRR and British Ecological Society Large Research Grant (5599-6643) to SAK. CNO was supported by a National Science Foundation Graduate Research Fellowship. QCW was supported by the Leadership Alliance Summer Research Early Identification Program.
Author contribution statement
SAK and CLW conceived the study and designed the experiments. CLW, SAK, QCW, and CNO conducted the experiments. SAK and JRR conducted the statistical analyses and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Communicated by Jean-François Le Galliard.
Rights and permissions
About this article
Cite this article
Knutie, S.A., Wilkinson, C.L., Wu, Q.C. et al. Host resistance and tolerance of parasitic gut worms depend on resource availability. Oecologia 183, 1031–1040 (2017). https://doi.org/10.1007/s00442-017-3822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3822-7