Abstract
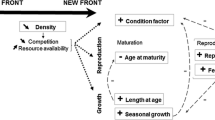
The colonisation of new environments is a central evolutionary process, yet why species make such transitions often remains unknown because of the difficulty in empirically investigating potential mechanisms. The most likely explanation for transitions to new environments is that doing so conveys survival benefits, either in the form of an ecological release or new ecological opportunity. Life history theory makes explicit predictions about how traits linked to survival and reproduction should change with shifts in age-specific mortality. We used these predictions to examine whether a current colonisation of land by fishes might convey survival benefits. We found that blenny species with more terrestrial lifestyles exhibited faster reproductive development and slower growth rates than species with more marine lifestyles; a life history trade off that is consistent with the hypothesis that mortality has become reduced in younger life stages on land. A plausible explanation for such a shift is that an ecological release or opportunity on land has conveyed survival benefits relative to the ancestral marine environment. More generally, our study illustrates how life history theory can be leveraged in novel ways to formulate testable predictions on why organisms might make transitions into novel environments.





Similar content being viewed by others
References
Almany GR, Webster MS (2006) The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25:19–22
Arbogast BS, Drovetski SV, Curry RL, Boag PT, Seutin G, Grant PR, Grant BR, Anderson DJ (2006) The origin and diversification of Galapagos mockingbirds. Evolution 60:370–382
Bates D (2008) lme4: linear mixed-effects models using S4 classes: R package, 0.999375–28. http://lme4.r-forge.r-project.org/
Belk MC (1995) Variation in growth and age at maturity in bluegill sunfish: genetic or environmental effects? J Fish Biol 47:237–247
Benard M (2004) Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Evol Syst 35:651–673
Berglund A, Rosenqvist G (1986) Reproductive costs in the prawn Palaemon adspersus: effects on growth and predator vulnerability. Oikos 46:349–354
Bhikajee M, Green JM (2002) Behaviour and habitat of the Indian Ocean amphibious blenny, Alticus monochrus. Afr Zool 37:221–230
Brockelman WY (1975) Competition, the fitness of offspring, and optimal clutch size. Am Nat 109:677–699
Brothers EB, Mathews CP, Lasker R (1976) Daily growth increments in otoliths from larval and adult fishes. Fish Bull 74:1–8
Brown CR, Gordon MS, Martin KLM (1992) Aerial and aquatic oxygen uptake in the amphibious red sea rockskipper fish, Alticus kirki (family Blenniidae). Copeia 1992:1007–1013
Campana SE, Neilson JD (1985) Microstructure of fish otoliths. Can J Fish Aquat Sci 42:1014–1032
Carlson BA (1992) The life history and reproductive success of the coral blenny, Exallias brevis (Kner, 1868). PhD thesis, University of Hawaii
Clelland ES, Tan Q, Balofsky A, Lacivita R, Peng C (2007) Inhibition of premature oocyte maturation: a role for bone morphogenetic protein 15 in zebrafish ovarian follicles. Endocrinology 148:5451–5458
Dabruzzi TF, Wygoda ML, Wright JE, Eme J, Bennett WA (2011) Direct evidence of cutaneous resistance to evaporative water loss in amphibious mudskipper (family Gobiidae) and rockskipper (family Blenniidae) fishes from Pulau Hoga, southeast Sulawesi, Indonesia. J Exp Mar Biol Ecol 405:125–129
Forrester GE (1990) Factors influencing the juvenile demography of a coral reef fish. Ecology 71:1666–1681
Gadgil M, Bossert WH (1970) Life historical consequences of natural selection. Am Nat 104:1–24
Geber MA (1990) The cost of meristem limitation in Polygonum arenastrum: negative genetic correlations between fecundity and growth. Evolution 44:799–819
Gibb AC, Ashley-Ross MA, Hsieh ST (2013) Thrash, flip, or jump: the behavioral and functional continuum of terrestrial locomotion in teleost fishes. Integr Comp Biol 53:295–306
Graham JB (1997) Air-breathing fishes: evolution, diversity and adaptation. Academic Press, San Diego
Graham JB, Lee HJ (2004) Breathing air in air: in what ways might extant amphibious fish biology relate to prevailing concepts about early tetrapods, the evolution of vertebrate air breathing, and the vertebrate land transition? Physiol Biochem Zool 77:720–731
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invas 10:483–506
Hernández-Miranda E, Veas R, Valeria Espinoza C, Thorrold SR, Ojeda FP (2009) The use of otoliths and larval abundance for studying the spatial ecology of the blenny Scartichthys viridis (Valenciennes, 1836) in coastal central Chile. Rev Biol Mar Oceanogr 44:619–633
Horn MH, Martin KLM, Chotkowski MA (eds) (1999) Intertidal fishes: life in two worlds. Academic Press, San Diego
Hsieh STT (2010) A locomotor innovation enables water-land transition in a marine fish. PLoS One 5:e11197
Hunte W, Côté IM (1989) Recruitment in the redlip blenny Ophioblennius atlanticus: is space limiting? Coral Reefs 8:45
Hutchings JA (1991) Fitness consequences of variation in egg size and food abundance in brook trout Salvelinus fontinalis. Evolution 45:1162–1168
Hutchings JA (1993) Adaptive life histories effected by age-specific survival and growth rate. Ecology 74:673–684
Jeffries MJ, Lawton JH (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Johansson F, Stoks R, Rowe L, de Block M (2001) Life history plasticity in a damselfly: effects of combined time and biotic constraints. Ecology 82:1857–1869
Knope ML, Scales JA (2013) Adaptive morphological shifts to novel habitats in marine sculpin fishes. J Evol Biol 26:472–482
Kornfield I, Smith PF (2000) African cichlid fishes: model systems for evolutionary biology. Annu Rev Ecol Syst 31:163–196
Law R (1979) Optimal life histories under age-specific predation. Am Nat 114:399–417
Lister BC (1976) The nature of niche expansion in West Indian Anolis lizards. I. Ecological consequences of reduced competition. Evolution 30:659–676
Losos JB, Mahler DL (2010) Adaptive radiation: the interaction of ecological opportunity, adaptation, and speciation. In: Bell MA, Futuyma DJ, Eanes WF, Levinton JS (eds) Evolution since Darwin: the first 150 years. Sinauer, Sunderland, pp 381–420
Martin KLM (1995) Time and tide wait for no fish: intertidal fishes out of water. Environ Biol Fish 44:165–181
Martin KLM (1996) An ecological gradient in air-breathing ability among marine cottid fishes. Physiol Zool 69:1096–1113
Martin KLM (2014) Theme and variations: amphibious air-breathing intertidal fishes. J Fish Biol 84:577–602
Martin KLM (2015) Beach-spawning fishes: reproduction in an endangered ecosystem. CRC Press, Boca Raton
Martin KLM, Carter AL (2013) Brave new propagules: terrestrial embryos in anamniotic eggs. Integr Comp Biol 53:233–247
Martin KLM, Lighton JRB (1989) Aerial CO2 and O2 exchange during terrestrial activity in an amphibious fish, Alticus kirki (Blenniidae). Copeia 1989:723–727
Martin KLM, Van Winkle RC, Drais JE, Lakisic H (2004) Beach spawning fishes, terrestrial eggs, and air breathing. Physiol Biochem Zool 77:750–759
Michod RE (1979) Evolution of life histories in response to age-specific mortality factors. Am Nat 113:531–550
Morgans CL, Ord TJ (2013) Natural selection in novel environments: predation selects for background matching in the body colour of a land fish. Anim Behav 86:1241–1249
Morgans CL, Cooke GM, Ord TJ (2014) How populations differentiate despite gene flow: sexual and natural selection drive phenotypic divergence within a land fish, the Pacific leaping blenny. BMC Evol Biol 14:97
Münzing J (1963) The evolution of variation and distributional patterns in European populations of the three-spined stickleback, Gasterosteus aculeatus. Evolution 17:320–332
Myers RA, Cadigan NG (1993) Density-dependent juvenile mortality in marine demersal fish. Can J Fish Aquat Sci 50:1576–1590
Nosil P (2012) Ecological speciation. Oxford University Press, Oxford
Núñez J, Duponchelle F (2009) Towards a universal scale to assess sexual maturation and related life history traits in oviparous teleost fishes. Fish Physiol Biochem 35:167–180
Ord TJ, Hsieh ST (2011) A highly social, land-dwelling fish defends territories in a constantly fluctuating environment. Ethology 117:918–927
Osenberg CW, Mittelbach GG, Wainwright PC (1992) Two-stage life histories in fish: the interaction between juvenile competition and adult performance. Ecology 73:255–267
Pace CM, Gibb AC (2014) Sustained periodic terrestrial locomotion in air-breathing fishes. J Fish Biol 84:1–22
Patzner RA, Gonçalves EJ, Hastings P, Kapoor BG (2009) The biology of blennies. CRC, Boca Raton
Pianka ER, Parker WS (1975) Age-specific reproductive tactics. Am Nat 109:453–464
Platt ERM, Ord TJ (2015) Population variation in the life history of a land fish, Alticus arnoldorum, and the effects of predation and density. PLoS One 10:e0137244
Rainey PB, Travisano M (1998) Adaptive radiation in a heterogeneous environment. Nature 394:69–72
Reznick DN, Bryga H (1987) Life-history evolution in guppies (Poecilia reticulata). 1. phenotypic and genetic changes in an introduction experiment. Evolution 41:1370–1385
Reznick D, Endler JA (1982) The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36:160–177
Reznick D, Bryga H, Endler JA (1990) Experimentally induced life-history evolution in a natural population. Nature 346:357–359
Robinson BW, Doyle RW (1985) Trade-off between male reproduction (amplexus) and growth in the amphipod Gammarus lawrencianus. Biol Bull 168:482–488
Rozemeijer MJC, Plaut I (1993) Regulation of nitrogen excretion of the amphibious Blenniidae Alticus kirki (Guenther, 1868) during emersion and immersion. Comp Biochem Physiol B 104A:57–63
Rundle HD, Schluter D (2004) Natural selection and ecological speciation in sticklebacks. In: Dieckmann U, Doebeli M, Metz JAJ, Tautz D (eds) Adaptive speciation. Cambridge University Press, Cambridge, pp 192–209
Ryser J (1989) Weight loss, reproductive output, and the cost of reproduction in the common frog, Rana temporaria. Oecologia 78:264–268
Sayer MDJ (2005) Adaptations of amphibious fish for surviving life out of water. Fish Fish 6:186–211
Sayer MDJ, Davenport J (1991) Amphibious fish: why do they leave water? Rev Fish Biol Fish 1:159–181
Schluter D (2009) Evidence for ecological speciation and its alternative. Science 323:737–741
Schwarzkopf L (1993) Costs of reproduction in water skinks. Ecology 74:1970–1981
Selman K, Wallace RA, Sarka A, Qi X (1993) Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morph 218:203–224
Shimizu N, Sakai Y, Hashimoto H, Gushima K (2006) Terrestrial reproduction by the air-breathing fish Andamia tetradactyla (Pisces; Blenniidae) on supralittoral reefs. J Zool 269:357–364
Sibly R, Calow P (1983) An integrated approach to life-cycle evolution using selective landscapes. J Theor Biol 102:527–547
Shulman MJ (1985) Recruitment of coral reef fishes: effects of distribution of predators and shelter. Ecology 66:1056–1066
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Sol D, Maspons J, Vall-llosera M, Bartomeus I, Garcia-Pena GE, Pinol J, Freckelton RP (2012) Unraveling the life history of successful invaders. Science 337:580–583
Stearns SC (1976) Life-history tactics: a review of the ideas. Quart Rev Biol 51:3–47
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Streelman JT, Danley PD (2003) The stages of vertebrate evolutionary radiation. Trends Ecol Evol 18:126–131
Sunobe T, Ohta T, Nakazono A (1995) Mating system and spawning cycle in the blenny, Istiblennius enosimae, at Kagoshima, Japan. Environ Biol Fish 43:195–199
Taylor HM, Gourley RS, Lawrence CE, Kaplan RS (1974) Natural selection of life history attributes: an analytical approach. Theoret Pop Biol 5:104–122
Tyler CR, Sumpter JP (1996) Oocyte growth and development in teleosts. Rev Fish Biol Fish 6:287–318
Vermeij GJ, Dudley R (2000) Why are there so few evolutionary transitions between aquatic and terrestrial ecosystems? Biol J Linn Soc 70:541–554
Victor BC (1986) Larval settlement and juvenile mortality in a recruitment-limited coral reef fish population. Ecol Monogr 56:145–160
Wilson EO (1961) The nature of the taxon cycle in the melanesian ant fauna. Am Nat 95:169–193
Wilson SK (2004) Growth, mortality and turnover rates of a small detritivorous fish. Mar Ecol Prog Ser 284:253–259
Yoder JB, Clancey E, Des Roches S, Eastman JM, Gentry L, Godsoe W, Hagey TJ, Jochimsen D, Oswarld BP, Robertson J, Sarver BAJ, Schenk JJ, Spear SF, Harmon LJ (2010) Ecological opportunity and the origin of adaptive radiations. J Evol Biol 23:1581–1596
Acknowledgments
We would like to thank Alex Kerr and Brett Taylor for logistical support on Guam, Georgina Cooke for assistance in specimen collection, Iain Suthers for the use of his lab equipment and David Chapple and several anonymous reviewers for comments on previous versions of this paper. This work was supported by an Australian Research Council grant to T. J. O., and a postgraduate research award from the School of Biological, Earth and Environmental Sciences to E. R. M. P. This study was covered by the University of New South Wales Animal Care and Ethics Committee protocol 11/36b initially approved on 10 March 2011 and most recently reviewed on 28 February 2013. All data from this publication have been archived in the Dryad Digital Repository (doi:10.5061/dryad.55f35). The authors declare no conflict of interest.
Author contribution statement
T. J. O. conceived the project. E. R. M. P. collected the data and performed the analyses. A. M. F. contributed to processing specimens. E. R. M. P., A. M. F. and T. J. O. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steve Swearer.
Highlighted student paper We use the study of life history in a novel way to investigate the potential consequences of the colonisation of a novel habitat. Much has been made of how transitions into new environments promote adaptive evolution and speciation but our understanding of why organisms make these transitions in the first place is far more limited. Our study provides a unique perspective on this question by leveraging an extraordinary vertebrate example of a major ecological transition: the colonisation of land by marine fish.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Platt, E.R.M., Fowler, A.M. & Ord, T.J. Land colonisation by fish is associated with predictable changes in life history. Oecologia 181, 769–781 (2016). https://doi.org/10.1007/s00442-016-3593-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3593-6