Abstract
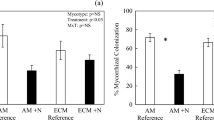
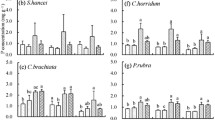
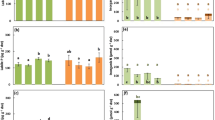
Plants on infertile soils exhibit physiological and morphological traits that support conservative internal nutrient cycling. However, potential trade-offs among use efficiencies for N, P, and cations are not well explored in species-rich habitats where multiple elements may limit plant production. We examined uptake efficiency and use efficiency of N, P, K, Ca, Mg, Al, and Na in plots of regenerating tropical dry forests spanning a gradient of soil fertility. Our aim was to determine whether plant responses to multiple elements are correlated, or whether there are trade-offs among exploitation strategies across stands varying in community composition, soil quality, and successional stage. For all elements, both uptake efficiency and use efficiency decreased as availability of the corresponding element increased. Plant responses to N, Na, and Al were uncoupled from uptake and use efficiencies for P and essential base cations, which were tightly correlated. N and P use efficiencies were associated with shifts in plant species composition along the soil fertility gradient, and there was also a trend towards increasing N use efficiency with stand age. N uptake efficiency was positively correlated with the abundance of tree species that associate with ectomycorrhizal fungi. Taken together, our results suggest that successional processes and local species composition interact to regulate plant responses to availability of multiple resources. Successional tropical dry forests appear to employ different strategies to maximize response to N vs. P and K.


Similar content being viewed by others
References
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Ashton I, Miller A, Bowman W, Suding K (2010) Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology 91:3252–3260
Averill C, Finzi A (2011) Increasing plant use of organic nitrogen with elevation is reflected in nitrogen uptake rates and ecosystem δ15N. Ecology 92:883–891
Bazzaz FA, Pickett STA (1980) Physiological ecology of tropical succession: a comparative review. Annu Rev Ecol Syst 11:287–310
Becknell JM, Powers JS (2014) Stand age and soils as drivers of plant functional traits and aboveground biomass in secondary tropical dry forest. Can J For Res 44:603–614
Bloom AJ, Chapin FS III, Mooney HA (1985) Resource limitation in plants—an economic analogy. Annu Rev Ecol Syst 16:363–392
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M et al (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59
Bridgham S, Pastor J, McClaugherty C, Richardson C (1995) Nutrient-use efficiency: a litterfall index a model and a test along a nutrient-availability gradient in North Carolina peatlands. Am Nat 145:1–21
Chapin FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Chrispeels M, Crawford N, Schroeder J (1999) Proteins for transport of water and mineral nutrients across the membranes of plant cells. Plant Cell 11:661–675
Clark R, Zeto S (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr 23:867–902
Cleveland C, Townsend AR, Schmidt SK, Constance BC (2003) Soil microbial dynamics and biogeochemistry in tropical forests and pastures southwestern Costa Rica. Ecol Appl 13:314–326
Cleveland C, Townsend A, Taylor P, Alvarez-Clare S, Bustamante M, Chuyong G et al (2011) Relationships among net primary productivity nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol Lett 14:939–947
Cooperband LR, Logan TJ (1994) Measuring in situ changes in labile soil phosphorus with anion-exchange membranes. SSSAJ 58:105–114
Davidson E, de Carvalho C, Vieira I, Figueiredo R, Moutinho P, Yoko Ishida F et al (2004) Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecol Appl 14:150–163
Davidson E, De Carvalho C, Figueira A, Ishida F, Ometto J, Nardoto G et al (2007) Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 447:995–998
do Rosario-Oliveira M, van Noordwijk M, Gaze SR, Brouwer G, Bona S et al (2000) Auger sampling ingrowth cores and pinboard methods. In: Smit AL, Bengough AG, Engels C, van Noordwijk M, Pellerin S, van de Geijn SC (eds) Root methods: a handbook. Springer, Berlin, pp 176–206
Eamus D (1999) Ecophysiological traits of deciduous and evergreen woody species in the seasonally dry tropics. Trends Ecol Evol 14:11–16
Farrior C, Tilman D, Dybzinski R, Reich P, Levin S, Pacala S (2013) Resource limitation in a competitive context determines complex plant responses to experimental resource additions. Ecology 94:2505–2517
Gahoonia T, Claassen N, Jungk A (1992) Mobilization of phosphate in different soils by ryegrass supplied with ammonium or nitrate. Plant Soil 140:241–248
Gotsch S, Powers J, Lerdau M (2010) Leaf traits and water relations of 12 evergreen species in Costa Rican wet and dry forests: patterns of intra-specific variation across forests and seasons. Plant Ecol 211:133–146
Gower ST, McMurtrie RE, Murty D (1996) Aboveground net primary production decline with stand age: potential causes. Trends Ecol Evol 11:378–382
Gray JT, Schlesinger WH (1983) Nutrient use by evergreen and deciduous shrubs in southern California. J Ecol 71:43–56
Harpole W, Ngai J, Cleland E, Seabloom E, Borer E, Bracken M et al (2011) Nutrient co-limitation of primary producer communities. Ecol Lett 14:852–862
Hayes P, Turner B, Lambers H, Laliberte E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410
Hietz H, Turner BL, Wanek W, Richter A, Nock CA, Wright SJ (2011) Long–term change in the nitrogen cycle of tropical forests. Science 334:664–666
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root—mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Jackson L, Burger M, Cavagnaro T (2008) Roots nitrogen transformations and ecosystem services. Annu Rev Plant Biol 59:341–363
Kaye JP, Hart SC (1997) Competition for nitrogen between plants and soil microorganisms. Trends Ecol Evol 12:139–142
Kissing L, Powers J (2010) Coarse woody debris stocks as a function of forest type and stand age in Costa Rican tropical dry forest: long-lasting legacies of previous land use. J Trop Ecol 26:467–471
Kitayama KE, Schuur AG, Drake DR, Mueller-Dombois D (2000) Soil phosphorus fractionation and phosphorus–use efficiencies of tropical rain forests along altitudinal gradients of Mount Kinabalu, Borneo. Oecologia 123:342–349
Knops J, Koenig W, Nash T III (1997) On the relationship between nutrient use efficiency and fertility in forest ecosystems. Oecologia 110:550–556
Liu K, Luan S (2001) Internal aluminum block of plant inward K+ channels. Plant Cell 13:1453–1466
Lü XT, Reed S, Yu Q, He NP, Wang ZW, Han XG (2013) Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiarid grassland. Glob Change Biol 19:2775–2784
Lynch J (1995) Root architecture and nutrient acquisition. Plant Physiol 109:7–13
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB et al (2013) Vegan: community ecology package for R
Ordoñez J, Van Bodegom P, Witte JP, Wright I, Reich P, Aerts R (2009) A global study of relationships between leaf traits climate and soil measures of nutrient fertility. Global Ecol Biogeogr 18:137–149
Ouedraogo DY, Mortier F, Gourlet-Fleury S, Freycon V, Picard N (2013) Slow-growing species cope best with drought: evidence from long-term measurements in a tropical semi-deciduous moist forest of Central Africa. J Ecol 101:1459–1470
Paoli G, Curran L, Zak D (2005) Phosphorus efficiency of Bornean rain forest productivity: evidence against the unimodal efficiency hypothesis. Ecology 86:1548–1561
Pastor J, Bridgham S (1999) Nutrient efficiency along nutrient availability gradients. Oecologia 118:50–58
Phillips R, Brzostek E, Midgley M (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol 199:41–51
Poorter L, Bongers L, Bongers F (2006) Architecture of 54 moist–forest tree species: traits trade-offs and functional groups. Ecology 87:1289–1301
Powers J, Peréz-Aviles D (2013) Edaphic factors are a more important control on surface fine roots than stand age in secondary tropical dry forests. Biotropica 45:1–9
Powers J, Tiffin P (2010) Plant functional type classification in tropical dry forests in Costa Rica: leaf habit versus taxonomic approaches. Funct Ecol 24:927–936
Powers J, Becknell J, Irving J, Perez-Aviles D (2009) Diversity and structure of regenerating tropical dry forests in Costa Rica: geographic patterns and environmental drivers. For Ecol Manage 258:959–970
Qian P, Schoenau JJ (1995) Assessing nitrogen mineralization from soil organic matter using anion exchange membranes. Fertil Res 40:143–148
Reed S, Townsend A, Davidson E, Cleveland C (2012) Stoichiometric patterns in foliar nutrient resorption across multiple scales. New Phytol 196:173–180
Reich P (2014) The worldwide fast–slow plant economics spectrum: a traits manifesto. J Ecol 102:275–301
Schimel J, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Silver W (1994) Is nutrient availability related to plant nutrient use in humid tropical forests? Oecologia 98:336–343
Smil V (2000) Phosphorus in the environment: natural flows and human interfaces. Annu Rev Energy Environ 25:53–88
ten Hoopen F, Cuin T, Pedas P, Hegelund J, Shabala S, Schjoerring J et al (2010) Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J Exp Bot 61:2303–2315
Tilman D, Kilham S, Kilham P (1982) Phytoplankton community ecology: the role of limiting nutrients. Annu Rev Ecol Syst 13:349–372
Townsend A, Cleveland C, Houlton B, Alden C, White J (2011) Multi–element regulation of the tropical forest carbon cycle. Front Ecol Environ 9:9–17
van Breugel M, Ransijn J, Craven D, Bongers F, Hall JS (2011) Estimating carbon stock in secondary forests: decisions and uncertainties associated with allometric biomass models. For Ecol Manage 262:1648–1657
Vitousek P (1982) Nutrient cycling and nutrient use efficiency. Am Nat 65:285–298
Vitousek P (1984) Litterfall nutrient cycling and nutrient limitation in tropical forests. Ecology 65:285–298
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW et al (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Wedin D, Tilman D (1993) Competition among grasses along a nitrogen gradient: initial conditions and mechanisms of competition. Ecol Monogr 63:199–229
Whiteside M, Treseder K, Atsatt P (2009) The brighter side of soils: quantum dots track organic nitrogen through fungi and plants. Ecology 90:100–108
Wright S, Yavitt J, Wurzburger N, Turner B, Tanner E, Sayer E et al (2011) Potassium phosphorus or nitrogen limit root allocation tree growth or litter production in a lowland tropical forest. Ecology 92:1616–1625
Acknowledgments
We thank Roger Blanco and Maria Marta Chavarria of the Área de Conservación Guanacaste for logistical support and Daniel Peréz-Aviles for excellent help in the field. We also thank two anonymous reviewers for comments that improved this paper. This study was funded by National Science Foundation CAREER grant DEB-1053237 to J. S. P.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hermann Heilmeier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Waring, B.G., Becknell, J.M. & Powers, J.S. Nitrogen, phosphorus, and cation use efficiency in stands of regenerating tropical dry forest. Oecologia 178, 887–897 (2015). https://doi.org/10.1007/s00442-015-3283-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3283-9