Abstract
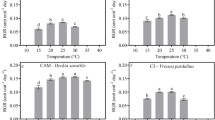
Two hypotheses—that elevated night-time temperatures due to climate warming would enforce post-fire dormancy of Proteaceae seed due to low moisture, and that periods without rain during summer would exceed desiccation periods tolerated by Proteaceae seedlings—were tested empirically. Enforced dormancy, i.e., the inability to germinate due to an environmental restraint, was tested by measuring seed germination in 11 Proteaceae species in experimental mesocosms whose soils were artificially elevated by 1.4 and 3.5 °C above ambient by far-red wavelength filtered infrared lamps. Diminished totality of germination and velocities were observed in 91 and 64 %, respectively, of the Proteaceae species tested. Drought resilience was tested in one-year-old seedlings of 16 Proteaceae species by withholding water from potted plants during summer in a greenhouse. The most drought-resilient Proteaceae species displayed the lowest initial transpiration rates at field capacity, the smallest declines in transpiration rate with decreasing soil water content, and the lowest water losses by transpiration. Projected drought periods leading to the complete cessation of transpiration in all Proteaceae species greatly exceeded the number of days without rain per month during summer in the current distribution ranges of those species. It was therefore concluded that enforced seed dormancy induced by elevated night-time temperatures is the post-fire recruitment stage of Proteaceae that is most sensitive to climate warming.






Similar content being viewed by others
References
Agam N, Berliner PR (2006) Dew formation and water-vapour adsorption in semi-arid environments––a review. J Arid Environ 65:572–590
Bates LM, Hall AE (1981) Stomatal closure with soil moisture depletion not associated with changes in bulk water status. Oecologia 50:62–65
Bond WJ (1988) Proteas as “tumbleseeds”: wind dispersal through the air and over soil. S Afr J Bot 54:455–460
Bond WJ, van Wilgen BW (1996) Fire and plants. Chapman and Hall, London, p 263
Brits GJ (1986) Influence of fluctuating temperatures and H2O2 treatment on germination of Leucospermum cordifolium and Serruria florida (Proteaceae) seeds. S Afr J Bot 52:286–290
Brits GJ (1987) Germination depth versus temperature requirements in naturally dispersed seeds of Leucospermum cordifolium and L. cuneiforme (Proteaceae). S Afr J Bot 53:119–124
Brits GJ, Van Niekerk MN (1986) Effects of air temperature, oxygenating treatments and low storage temperature on seasonal germination response of Leucospermum cordifolium (Proteaceae) seeds. S Afr J Bot 52:207–211
Brits GJ, Calitz FJ, Brown NAC, Manning JC (1993) Desiccation as the active principle in heat-stimulated seed germination of Leucospermum R.Br. (Proteaceae) in fynbos. New Phytol 125:397–403
Brown NAC, Botha PA (2004) Smoke seed germination studies and a guide to seed propagation of plants from the major families of the Cape Floristic Region, South Africa. S Afr J Bot 70:559–581
Chandra A, Pathak PS, Bhatt RK, Dubey A (2004) Variation in drought tolerance of different Stylosanthes accessions. Biol Plant 48:457–460
Chapotin SM, Razanameharizaka JH, Michele Holbrook N (2006) Water relations of baobab trees (Adansonia spp L.) during the rainy season: does stem water buffer daily water deficits? Plant Cell Environ 21:1021–1032
Chaves MM, Pereira JS, Maroco J, Rodriques ML, Ricardo CPP, Osorio ML, Carvatho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field photosynthesis and growth? Ann Bot 89:907–916
Cornelius JM, Kemp PR, Ludwig JA, Cunningham GL (1991) The distribution of vascular plant species and guilds in space and time along a desert gradient. J Veg Sci 2:59–72
Cowling RM, Campbell BM (1983) The definition of leaf consistence categories in the fynbos biome and their distribution along an altitudinal gradient in the South Eastern Cape. S Afr J Bot 49:87–101
Cowling RM, Rundel PW, Desmet PG, Esler KJ (1998) Extra ordinarily high regional-scale plant diversity in southern African arid lands: sub-continental and global comparisons. Divers Distrib 4:27–36
Czabator FJ (1962) Germination value: an index combining speed and completeness of pine seed germination. For Sci 8:386–395
Czarnomski NM, Moore GW, Pypker TG, Licata J, Bond BJ (2005) Precision and accuracy of three alternative soil water content instruments in two forest soils of the Pacific Northwest. Can J For Res 35:1867–1876
Daws MI, Davies J, Vaes E, van Gelder R, Pritchard HW (2007) Two-hundred-year seed survival of Leucospermum and two other woody species from the Cape Floristic region, South Africa. Seed Sci Res 17:73–79
Deall GB, Brown NAC (1981) Seed germination in Protea magnifica Link. S Afr J Sci 77:175–176
Esler KJ, Phillips N (1994) Experimental effects of water stress on semi-arid Karoo seedlings: implication for seedling survivorship. J Arid Environ 26:325–337
Francis M, Fey M, Prinsloo H, Ellis F, Mills A, Medinski T (2007) Soils of Namaqualand: compensations of aridity. J Arid Environ 70:588–603
Groom PK, Lamont BB (1997) Xerophytic implications of increased sclerophylly: interactions with water and light in Hakea psilorryncha seedlings. New Phytol 136:231–237
Hammill KA, Bradstock RA, Allaway WG (1998) Post-fire seed dispersal and species re-establishment in proteaceous heath. Aust J Bot 46:407–419
Harrison AT, Small E, Mooney HA (1971) Drought relationships and distribution of two Mediterranean-climate California shrubs. Ecology 52:869–875
Heelemann S, Proches S, Rebelo AG, Van Wilgen B, Porembski S, Cowling RM (2008) Fire season effects on the recruitment of non-sprouting serotinous Proteaceae in the eastern (bimodal rainfall) fynbos biome, South Africa. Austral Ecol 33:119–127
Hoffman MT, Carrick PJ, Gillson L, West AG (2009) Drought, climate change and vegetation response in the succulent Karoo, South Africa. S Afr J Sci 105:54–60
Hulme M, Doherty R, Ngara T, New M, Lister D (2001) African climate change: 1900-2100. Clim Res 17:145–168
IPCC (2007) Climate change 2007: the scientific basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jones HG (1998) Stomatal control of photosynthesis and transpiration. J Exp Bot 49:387–398
Jones HG, Tardieu F (1998) Modelling water relations of horticultural crops: a review. Sci Hortic 74:21–46
Klinkhamer PGL, de Jong TJ (1988) The importance of small scale disturbance for seedling establishment in Cirsium vulgare and Cynoglossum officinale. J Ecol 76:383–392
Kramer PJ (1988) Changing concepts regarding plant water relations. Plant Cell Environ 11:565–568
Kruger FJ, Bigalke RC (1984) Fire in fynbos. In: de Booysen P, Tainton NM (eds) Ecological effects of fire in South African ecosystems. Ecol Stud vol 48. Springer, Berlin, pp 67–114
Landhausser SM, Wein RW (1994) Post fire vegetation recovery and tree establishment at the Arctic tree line: climate-change-vegetation-response hypothesis. J Ecol 81:665–672
Lynch SD, Schulze (2007) Rainfall database (Section 2.2). In: Schulze RE (ed) South African atlas of climatology and agrohydrology. WRC report 1489/1/06. Water Research Commission, Pretoria
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. South African National Biodiversity Institute, Pretoria
Mustart PJ, Cowling RM (1993a) The role of regeneration stages in the distribution of edaphically restricted fynbos Proteaceae. Ecology 74:1490–1499
Mustart PJ, Cowling RM (1993b) Effects of soil and seed characteristics on seed germination and their possible roles in determining field emergence patterns of four Agulhas Plain (South Africa) Proteaceae. Can J Bot 71:1363–1368
Mustart PJ, Rebelo AG, Juritz J, Cowling RM (2012) Wide variation in post-emergence desiccation tolerance of seedlings of fynbos proteoid shrubs. S Afr J Bot 80:110–117
Niinemets Ü (2001) Global-Scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469
Ooi MK, Auld TD, Whelan RJ (2006) Dormancy and the fire-centric focus. Germination of three Leucopogon species (Ericaceae) from south-eastern Australia. Ann Bot 98:421–430
Paul ND, Jacobson NJ, Taylor A, Wargent JJ, Moore JP (2005) The use of wavelength-selective plastic cladding materials in horticulture: understanding of crop and fungal responses through the assessment of biological spectral weighting functions. Photochem Photobiol 81:1052–1060
Petit JR, Juozel J, Raynaud D, Barkov NI, Barnola J-M, Basile I, Bender M, Chappellaz J, Davis M, Delaygue G, Delmotte M, Kotalyakov VM, Legrand M, Lipenkov VY, Lorius C, Pépin L, Ritz C, Saltzman E, Stievenard M (1999) Climate and atmospheric history from the past 420,000 years from the Vostok ice core, Antarctica. Nature 399:429–436
Rebelo T (2010) Sasol proteas––a field guide to the proteas of Southern Africa, revised edn. Fernwood, Vlaeberg
Salleo S, Lo Gullo MA (1990) Sclerophylly and plant water relations in three Mediterranean Quercus species. Ann Bot 65:259–270
Schulze RE, Horan MJC (2007) Altitude and relative relief (Section 3.1). In: Schulze RE (ed) South African atlas of climatology and agrohydrology. WRC report 1489/1/06. Water Research Commission, Pretoria
Schulze RE, Maharaj M (2007) Temperature database (Section 2.1). In: Schulze RE (ed) South African atlas of climatology and agrohydrology. WRC report 1489/1/06. Water Research Commission, Pretoria
Schurr FM, Bond WJ, Midgley GF, Higgins SI (2005) A mechanistic model for secondary seed dispersal by wind and its experimental validation. J Ecol 93:1017–1028
Slingsby P, Bond WJ (1985) The influence of ants on the dispersal distance and recruitment of Leucospermum conocarpodendron (L.) Buek (Proteaceae). S Afr J Bot 51:30–34
Tardieu F, Zhang J, Gowing DJC (1993) Stomatal control by both [ABA] in the xylem sap and leaf water status: test of a model and alternative hypotheses for droughted or ABA-fed field-grown maize. Plant Cell Environ 16:413–420
Valente L, Reeves G, Schnitzler J, Mason IP, Fay MF, Rebelo TG, Chase MW, Barraclough TG (2010) Diversification of the African genus Protea (Proteaceae) in the Cape biodiversity hotspot and beyond: equal rates in different biomes. Evolution 64:745–760
Yin X (2002) Responses of leaf nitrogen concentration and specific leaf area to atmospheric CO2 enrichment: a retrospective synthesis across 62 species. Glob Change Biol 8:631–642
Acknowledgments
We wish to sincerely thank Mr. L. Powrie for extracting Proteaceae species distributions and environmental records from various databases, and Mr. S. Snyders for assisting with the construction of experimental warming mesocosms and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Allan T. G. Green.
Rights and permissions
About this article
Cite this article
Arnolds, J.L., Musil, C.F., Rebelo, A.G. et al. Experimental climate warming enforces seed dormancy in South African Proteaceae but seedling drought resilience exceeds summer drought periods. Oecologia 177, 1103–1116 (2015). https://doi.org/10.1007/s00442-014-3173-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3173-6