Abstract
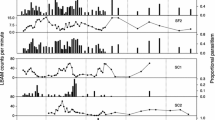
Many populations of forest Lepidoptera exhibit 10-year cycles in densities, with impressive outbreaks across large regions. Delayed density-dependent interactions with natural enemies are recognized as key factors driving these cyclic population dynamics, but emphasis has typically been on the larval stages. Eggs, pupae and adults also suffer mortality from predators, parasitoids and pathogens, but little is known about possible density relationships between mortality factors and these non-feeding life stages. In a long-term field study, we experimentally deployed autumnal moth (Epirrita autumnata) eggs and pupae to their natural enemies yearly throughout the 10-year population cycle in northern Norway. The abundance of another geometrid, the winter moth (Operophtera brumata), increased in the study area, permitting comparisons between the two moth species in predation and parasitism. Survival of autumnal moth eggs and pupae was related to the moth abundance in an inverse and delayed manner. Egg and pupal parasitoids dominated as density-dependent mortality factors and predicted the subsequent growth rate of the host population size. In contrast, effects of egg and pupal predators were weakly density dependent, and generally predation remained low. Parasitism rates did not differ between the autumnal and winter moth pupae, whereas predators preferred winter moth pupae over those of the autumnal moth. We conclude that parasitism of the autumnal moth by egg and pupal parasitoids can be related to the changes of the moth density in a delayed density-dependent manner. Furthermore, egg and pupal parasitoids cannot be overlooked as causal factors for the population cycles of forest Lepidoptera in general.








Similar content being viewed by others
References
Ammunét T, Kaukoranta T, Saikkonen K, Repo T, Klemola T (2012) Invading and resident defoliators in a changing climate: cold tolerance and predictions concerning extreme winter cold as a range-limiting factor. Ecol Entomol 37:212–220. doi:10.1111/j.1365-2311.2012.01358.x
Ammunét T, Klemola T, Parvinen K (2014) Consequences of asymmetric competition between resident and invasive defoliators: a novel empirically based modelling approach. Theor Popul Biol 92:107–117. doi:10.1016/j.tpb.2013.12.006
Andersson M, Erlinge S (1977) Influence of predation on rodent populations. Oikos 29:591–597
Aukema JE, Leung B, Kovacs K, Chivers C, Britton KO, Englin J, Frankel SJ, Haight RG, Holmes TP, Liebhold AM, McCullough DG, Von Holle B (2011) Economic impacts of non-native forest insects in the continental US. PLoS One 6:e24587. doi:10.1371/journal.pone.0024587
Baltensweiler W, Benz G, Bovey P, Delucchi V (1977) Dynamics of larch bud moth populations. Annu Rev Entomol 22:79–100. doi:10.1146/annurev.en.22.010177.000455
Berryman AA (1996) What causes population cycles of forest Lepidoptera? Trends Ecol Evol 11:28–32. doi:10.1016/0169-5347(96)81066-4
Berryman AA (2002) Population cycles: the case for trophic interactions. Oxford University Press, New York
Berryman AA, Stenseth NC, Isaev AS (1987) Natural regulation of herbivorous forest insect populations. Oecologia 71:174–184. doi:10.1007/BF00377282
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Dwyer G, Dushoff J, Yee SH (2004) The combined effects of pathogens and predators on insect outbreaks. Nature 430:341–345. doi:10.1038/nature02569
East R (1974) Predation on the soil-dwelling stages of the winter moth at Wytham woods, Berkshire. J Anim Ecol 43:611–626
Elderd BD, Rehill BJ, Haynes KJ, Dwyer G (2013) Induced plant defenses, host–pathogen interactions, and forest insect outbreaks. Proc Natl Acad Sci USA 110:14978–14983. doi:10.1073/pnas.1300759110
Elkinton JS, Healy WM, Buonaccorsi JP, Boettner GH, Hazzard AM, Smith HR, Liebhold AM (1996) Interactions among gypsy moths, white-footed mice, and acorns. Ecology 77:2332–2342. doi:10.2307/2265735
Elkinton JS, Liebhold AM, Muzika RM (2004) Effects of alternative prey on predation by small mammals on gypsy moth pupae. Popul Ecol 46:171–178. doi:10.1007/s10144-004-0175-y
Elton C (1924) Periodic fluctuations in the numbers of animals: their causes and effects. Br J Exp Biol 2:119–163
Frank JH (1967a) The effect of pupal predators on a population of winter moth, Operopthera brumata (L.) (Hydriomenidae). J Anim Ecol 36:611–621
Frank JH (1967b) The insect predators of the pupal stage of the winter moth, Operopthera brumata (L.) (Lepidoptera: Hydriomenidae). J Anim Ecol 36:375–389
Graham MH (2003) Confronting multicollinearity in ecological multiple regression. Ecology 84:2809–2815. doi:10.1890/02-3114
Hagen SB, Jepsen JU, Schott T, Ims RA (2010) Spatially mismatched trophic dynamics: cyclically outbreaking geometrids and their larval parasitoids. Biol Lett 6:566–569. doi:10.1098/rsbl.2009.1002
Hansen NM, Ims RA, Hagen SB (2009) No impact of pupal predation on the altitudinal distribution of autumnal moth and winter moth (Lepidoptera: Geometridae) in sub-arctic birch forest. Environ Entomol 38:627–632. doi:10.1603/022.038.0313
Haukioja E, Neuvonen S, Hanhimäki S, Niemelä P (1988) The autumnal moth in Fennoscandia. In: Berryman AA (ed) Dynamics of forest insect populations: patterns, causes, and implications. Plenum, London, pp 163–178
Haynes KJ, Liebhold AM, Fearer TM, Wang G, Norman GW, Johnson DM (2009) Spatial synchrony propagates through a forest food web via consumer–resource interactions. Ecology 90:2974–2983. doi:10.1890/08-1709.1
Hegazi EM, Khafagi WE (2000) Possible bases of pseudoparasitism in Spodoptera littoralis larvae stung by Microplitis rufiventris. J Insect Physiol 46:1267–1274. doi:10.1016/S0022-1910(00)00047-0
Heisswolf A, Klemola N, Ammunét T, Klemola T (2009) Responses of generalist invertebrate predators to pupal densities of autumnal and winter moths under field conditions. Ecol Entomol 34:709–717. doi:10.1111/j.1365-2311.2009.01121.x
Hogstad O (1997) Population fluctuations of Epirrita autumnata Bkh. and Operophtera brumata (L.) (Lep., Geometridae) during 25 years and habitat distribution of their larvae during a mass outbreak in a subalpine birch forest in Central Norway. Fauna Nor Ser B 44:1–10
Jepsen JU, Hagen SB, Ims RA, Yoccoz NG (2008) Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J Anim Ecol 77:257–264. doi:10.1111/j.1365-2656.2007.01339.x
Jepsen JU, Biuw M, Ims RA, Kapari L, Schott T, Vindstad OPL, Hagen SB (2013) Ecosystem impacts of a range expanding forest defoliator at the forest-tundra ecotone. Ecosystems 16:561–575. doi:10.1007/s10021-012-9629-9
Kareiva P, Odell G (1987) Swarms of predators exhibit “preytaxis” if individual predators use area-restricted search. Am Nat 130:233–270
Kenis M, Herz K, West RJ, Shaw MR (2005) Parasitoid assemblages reared from geometrid defoliators (lepidoptera: Geometridae) of larch and fir in the Alps. Agric For Entomol 7:307–318. doi:10.1111/j.1461-9555.2005.00277.x
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Klemola T, Tanhuanpää M, Korpimäki E, Ruohomäki K (2002) Specialist and generalist natural enemies as an explanation for geographical gradients in population cycles of northern herbivores. Oikos 99:83–94. doi:10.1034/j.1600-0706.2002.990109.x
Klemola T, Klemola N, Andersson T, Ruohomäki K (2007) Does immune function influence population fluctuations and level of parasitism in the cyclic geometrid moth? Popul Ecol 49:165–178. doi:10.1007/s10144-007-0035-7
Klemola T, Andersson T, Ruohomäki K (2008) Fecundity of the autumnal moth depends on pooled geometrid abundance without a time lag: implications for cyclic population dynamics. J Anim Ecol 77:597–604. doi:10.1111/j.1365-2656.2008.01369.x
Klemola N, Heisswolf A, Ammunét T, Ruohomäki K, Klemola T (2009) Reversed impacts by specialist parasitoids and generalist predators may explain a phase lag in moth cycles: a novel hypothesis and preliminary field tests. Ann Zool Fenn 46:380–393. doi:10.5735/086.046.0504
Klemola N, Andersson T, Ruohomäki K, Klemola T (2010) Experimental test of parasitism hypothesis for population cycles of a forest lepidopteran. Ecology 91:2506–2513. doi:10.1890/09-2076.1
Klomp H (1966) The dynamics of a field population of the pine looper, Bupalus piniarius L. (Lep., Geom.). Adv Ecol Res 3:207–305. doi:10.1016/S0065-2504(08)60312-8
Liebhold A, Elkinton J, Williams D, Muzika RM (2000) What causes outbreaks of the gypsy moth in North America? Popul Ecol 42:257–266. doi:10.1007/PL00012004
MacPhee AW (1967) The winter moth, Operophtera Brumata (Lepidoptera: Geometridae), a new pest attacking apple orchards in Nova Scotia, and its cold hardiness. Can Entomol 99:829–834. doi:10.4039/Ent99829-8
May RM (1981) Models for single populations. In: May RM (ed) Theoretical ecology: principles and applications. Blackwell, Oxford, pp 5–29
Münster-Swendsen M (2002) The role of insect parasitoids in population cycles of the spruce needle miner in Denmark. In: Berryman AA (ed) Population cycles: the case for trophic interactions. Oxford University Press, New York, pp 29–43
Myers JH (1988) Can a general hypothesis explain population cycles of forest Lepidoptera? Adv Ecol Res 18:179–242. doi:10.1016/S0065-2504(08)60181-6
Myers JH, Cory JS (2013) Population cycles in forest Lepidoptera revisited. Annu Rev Ecol Evol Syst 44:565–592. doi:10.1146/annurev-ecolsys-110512-135858
Neuvonen S, Niemelä P, Virtanen T (1999) Climatic change and insect outbreaks in boreal forests: the role of winter temperatures. Ecol Bull 47:63–67
Nuessly GS, Sterling WL (1994) Mortality of Helicoverpa zea (Lepidoptera: Noctuidae) eggs in cotton as a function of oviposition sites, predator species, and desiccation. Environ Entomol 23:1189–1202
Raymond B, Vanbergen A, Watt A, Hartley SE, Cory JS, Hails RS (2002) Escape from pupal predation as a potential cause of outbreaks of the winter moth, Operophtera brumata. Oikos 98:219–228. doi:10.1034/j.1600-0706.2002.980204.x
Régnière J, Nealis VG (2007) Ecological mechanisms of population change during outbreaks of the spruce budworm. Ecol Entomol 32:461–477. doi:10.1111/j.1365-2311.2007.00888.x
Roland J (1988) Decline in winter moth populations in North America: direct versus indirect effect of introduced parasites. J Anim Ecol 57:523–531
Roland J (1990) Interaction of parasitism and predation in the decline of winter moth in Canada. In: Watt AD, Leather SR, Hunter MD, Kidd NAC (eds) Population dynamics of forest insects. Intercept, Andover, pp 289–302
Roland J (1994) After the decline: what maintains low winter moth density after successful biological control? J Anim Ecol 63:392–398
Royama T (1984) Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol Monogr 54:429–462. doi:10.2307/1942595
Ruohomäki K (1994) Larval parasitism in outbreaking and non-outbreaking populations of Epirrita autumnata (Lepidoptera, Geometridae). Entomol Fenn 5:27–34
Ruohomäki K, Virtanen T, Kaitaniemi P, Tammaru T (1997) Old mountain birches at high altitudes are prone to outbreaks of Epirrita autumnata (Lepidoptera: Geometridae). Environ Entomol 26:1096–1104
Ruohomäki K, Tanhuanpää M, Ayres MP, Kaitaniemi P, Tammaru T, Haukioja E (2000) Causes of cyclicity of Epirrita autumnata (Lepidoptera, Geometridae): grandiose theory and tedious practice. Popul Ecol 42:211–223. doi:10.1007/PL00012000
Schott T, Hagen SB, Ims RA, Yoccoz NG (2010) Are population outbreaks in sub-arctic geometrids terminated by larval parasitoids? J Anim Ecol 79:701–708. doi:10.1111/j.1365-2656.2010.01673.x
Schott T, Ims RA, Hagen SB, Yoccoz NG (2012) Sources of variation in larval parasitism of two sympatrically outbreaking birch forest defoliators. Ecol Entomol 37:471–479. doi:10.1111/j.1365-2311.2012.01386.x
Stroup WW (2013) Generalized linear mixed models: modern concepts, methods and applications. CRC, Boca Raton
Tanhuanpää M, Ruohomäki K, Kaitaniemi P, Klemola T (1999) Different impact of pupal predation on populations of Epirrita autumnata (Lepidoptera: Geometridae) within and outside the outbreak range. J Anim Ecol 68:562–570. doi:10.1046/j.1365-2656.1999.00305.x
Tanhuanpää M, Ruohomäki K, Turchin P, Ayres MP, Bylund H, Kaitaniemi P, Tammaru T, Haukioja E (2002) Population cycles of the autumnal moth in Fennoscandia. In: Berryman AA (ed) Population cycles: the case for trophic interactions. Oxford University Press, New York, pp 142–154
Tanhuanpää M, Ruohomäki K, Kaitaniemi P (2003) Influence of adult and egg predation on reproductive success of Epirrita autumnata (Lepidoptera: Geometridae). Oikos 102:263–272. doi:10.1034/j.1600-0706.2003.12546.x
Teder T, Tanhuanpää M, Ruohomäki K, Kaitaniemi P, Henriksson J (2000) Temporal and spatial variation of larval parasitism in non-outbreaking populations of a folivorous moth. Oecologia 123:516–524. doi:10.1007/s004420000346
Tenow O (1972) The outbreaks of Oporinia autumnata Bkh. and Operophthera spp. (Lep., Geometridae) in the Scandinavian mountain chain and northern Finland 1862–1968. Zool Bidr Uppsala Suppl 2:1–107
Tenow O, Bylund H, Holmgren B (2001) Impact on mountain birch forests in the past and the future of outbreaks of two geometrid insects. In: Wielgolaski FE (ed) Nordic mountain birch ecosystems. Unesco, Paris, pp 223–239
Tenow O, Nilssen AC, Bylund H, Hogstad O (2007) Waves and synchrony in Epirrita autumnata/Operophtera brumata outbreaks. I. Lagged synchrony: regionally, locally and among species. J Anim Ecol 76:258–268. doi:10.1111/j.1365-2656.2006.01204.x
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Princeton University Press, Princeton
Varley GC, Gradwell GR (1960) Key factors in population studies. J Anim Ecol 29:399–401
Vindstad OPL, Hagen SB, Schott T, Ims RA (2010) Spatially patterned guild structure in larval parasitoids of cyclically outbreaking winter moth populations. Ecol Entomol 35:456–463. doi:10.1111/j.1365-2311.2010.01201.x
Acknowledgments
We thank all field assistants for their great help with the fieldwork. Ph.D. students and post doc researchers of the earlier autumnal moth projects, Tea Ammunét, Netta Klemola and Annette Scheiner, are especially acknowledged. Reijo Jussila, Ilari E. Sääksjärvi and Veli Vikberg are gratefully thanked for their help in parasitoid identification. We also thank Thomas Hoffmeister, Judith H. Myers, Seppo Neuvonen, Jens Roland and an anonymous referee for their valuable comments on the manuscript, the staff of the Kevo Subarctic Research Station for excellent working facilities and the staff of the Statskog for permissions to conduct our research on public lands in Norway. The research was supported financially by the Academy of Finland (projects 111195, 129143 and 204190 to T. K. and 7650, 34509 and 48697 to K. R.), by the Turku University Foundation (grants to T. K. and K. R.), and by the Maj and Tor Nessling Foundation (grants to T. K.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas S. Hoffmeister.
Electronic supplementary material
Below is the link to the electronic supplementary material.
442_2014_2984_MOESM1_ESM.docx
Online Resource 1 Map of northern Europe showing outbreak range of the autumnal and winter moth and main study locations of the moths in northern Fennoscandia (DOCX 124 kb)
442_2014_2984_MOESM2_ESM.docx
Online Resource 2 Likelihood-based model comparisons and parameter estimates of GLMMs on the survival, predation, and parasitism of autumnal moth eggs and pupae (DOCX 32 kb)
442_2014_2984_MOESM4_ESM.docx
Online Resource 4 Information on parasitoids and potential predators of the pupae and eggs of autumnal and winter moths (DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Klemola, T., Andersson, T. & Ruohomäki, K. Delayed density-dependent parasitism of eggs and pupae as a contributor to the cyclic population dynamics of the autumnal moth. Oecologia 175, 1211–1225 (2014). https://doi.org/10.1007/s00442-014-2984-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2984-9