Abstract
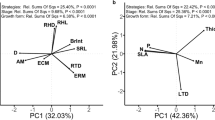
Several studies across species have linked leaf functional traits with shade tolerance. Because evolution by natural selection occurs within populations, in order to explain those interspecific patterns it is crucial to examine variation of traits associated with shade tolerance and plant fitness at an intraspecific scale. In a southern temperate rainforest, two climbing plant species coexist but differ in shade tolerance. Whereas Luzuriaga radicans is most abundant in the shaded understory, L. polyphylla typically occurs in intermediate light environments. We carried out an intraspecific approach to test the hypothesis of differential selection patterns in relation to shade tolerance in these congeneric species. The probability of showing reproductive structures increased with specific leaf area (SLA) in L. polyphylla, and decreased with dark respiration in L. radicans. When reproductive output of fertile individuals was the fitness variable, we detected positive directional selection on SLA in L. polyphylla, and negative directional selection on dark respiration and positive directional selection on leaf size in L. radicans. Total light radiation differed between the microsites where the Luzuriaga species were sampled in the old-growth forest understory. Accordingly, L. radicans had a lower minimum light requirement and showed fertile individuals in darker microsites. L. radicans showed lower dark respiration, higher chlorophyll content, and greater leaf size and SLA than L. polyphylla. Results suggest that in more shade-tolerant species, established in the darker microsites, selection would favor functional traits minimizing carbon losses, while in less shade-tolerant species, plants displaying leaf traits enhancing light capture would be selected.


Similar content being viewed by others
References
Abrams MD, Kubiske ME (1990) Leaf structural characteristics of 31 hardwood and conifer tree species in central Wisconsin: influence of light regime and shade tolerance rank. For Ecol Manag 31:245–253
Ackerly DD (2004) Adaptation, niche conservatism, and convergence: comparative studies of leaf evolution in the California Chaparral. Am Nat 163:654–671
Ackerly DD, Dudley SA, Sultan SE, Schmitt J, Coleman JS, Linder CR, Sandquist DR, Geber MA, Evans AS, Dawson TE, Lechowicz MJ (2000) The evolution of plant ecophysiological traits: recent advances and future directions. Bioscience 50:979–995
Agrawal AA, Erwin AC, Cook SC (2008) Natural selection on and predicted responses of ecophysiological traits of swamp milkweed (Asclepias incarnata). J Ecol 96:536–542
Aide TM, Zimmerman JK (1990) Patterns of insect herbivory, growth and survivorship in juveniles of a neotropical liana. Ecology 71:1412–1421
Armesto JJ, Rozzi R, Miranda P, Sabag C (1987) Plant/frugivore interactions in South American temperate forests. Rev Chil Hist Nat 60:321–336
Arntz AM, Delph LF (2001) Pattern and process: evidence for the evolution of photosynthetic traits in natural populations. Oecologia 127:455–467
Arroyo MTK, Humaña AM (1999) Breeding systems of two endemic rainforest species in Southern Chile: Amomyrtus meli (Phil.) Legr. et Kaus. (Myrtaceae) and Luzuriaga polyphylla (Hook.) Macbr. (Philesiaceae). Gayana Bot 56:31–37
Baars R, Kelly D, Sparrow AD (1998) Liana distribution within native forest remnants in two regions of the South Island New Zealand. NZ J Ecol 22:71–85
Baltzer JL, Thomas SC (2007) Determinants of whole-plant light requirements in Bornean rain forest tree saplings. J Ecol 95:1208–1221
Bazzaz FA (1996) Plants in changing environments. Linking physiological, population, and community ecology. Cambridge University Press, Cambridge
Bellow JG, Nair PKR (2003) Comparing common methods for assessing understory light availability in shaded-perennial agroforestry systems. Agric For Meteorol 114:197–211
Brodie ED, Moore AJ, Janzen FJ (1995) Visualizing and quantifying natural-selection. Trends Ecol Evol 10:313–318
Carrasco-Urra F, Gianoli E (2009) Abundance of climbing plants in a southern temperate rainforest: host-tree characteristics or light availability? J Veg Sci 20:1155–1162
Craine JM, Reich PB (2005) Leaf-level light compensation points in shade-tolerant woody seedlings. New Phytol 166:710–713
Davies SJ (1998) Photosynthetic characteristics of nine pioneer Macaranga species from Borneo in relation to life-history traits. Ecology 79:2292–2308
Dorsch K (2003) Hydrogeologische Untersuchungen der Geothermalfelder von Puyehue und Cordón Caulle, Chile. PhD dissertation, Ludwig-Maximilians-Universität, München, Germany
Dudley SA (1996) Differing selection on plant physiological traits to environmental water availability: a test of adaptive hypotheses. Evolution 50:92–102
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Farris MA, Lechowicz MJ (1990) Functional interactions among traits that determine reproductive success in a native annual plant. Ecology 71:548–557
Geber MA, Griffen LR (2003) Inheritance and natural selection on functional traits. Int J Plant Sci 164:S21–S42
Gentry AH (1991) The distribution and evolution of climbing plants. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge, pp 3–49
Gianoli E (2002) Maternal environmental effects on the phenotypic responses of the twining vine Ipomoea purpurea to support availability. Oikos 99:324–330
Gianoli E, Valladares F (2012) Studying phenotypic plasticity: the advantages of a broad approach. Biol J Linn Soc 105:1–7
Gianoli E, Saldaña A, Jiménez-Castillo M, Valladares F (2010) Distribution and abundance of vines along the light gradient in a southern temperate rainforest. J Veg Sci 21:66–73
Gianoli E, Saldaña A, Jiménez-Castillo M (2012) Ecophysiological traits may explain the abundance of climbing plant species across the light gradient in a temperate rainforest. PLoS ONE 7(6):e38831
Gilbert B, Wright SJ, Muller-Landau HC, Kitajima K, Hernández A (2006) Life history trade-offs in tropical trees and lianas. Ecology 87:1281–1288
Givnish TJ (1988) Adaptation to sun and shade—a whole-plant perspective. Aust J Plant Physiol 15:63–92
González-Teuber M, Gianoli E (2008) Damage and shade enhance climbing and promote associational resistance in a climbing plant. J Ecol 96:122–126
Grime JP, Jeffery DW (1965) Seedling establishment in vertical gradients of sunlight. J Ecol 53:621–642
Harvey PH, Read AF, Nee S (1995) Why ecologists need to be phylogenetically challenged. J Ecol 83:535–536
Heschel MS, Stinchcombe JR, Holsinger KE, Schmitt J (2004) Natural selection on light response curve parameters in the herbaceous annual, Impatiens capensis. Oecologia 139:487–494
Janse-ten Klooster SH, Thomas EJP, Sterck FJ (2007) Explaining interspecific differences in sapling growth and shade tolerance in temperate forests. J Ecol 95:1250–1260
Janzen FJ, Stern HS (1998) Logistic regression for empirical studies of multivariate selection. Evolution 52:1564–1571
Kitajima K (1994) Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226
Lusk CH (2002) Leaf area accumulation helps juvenile evergreen trees tolerate shade in a temperate rainforest. Oecologia 132:188–196
Lusk CH (2004) Leaf area and growth of juvenile temperate evergreens in low light: species of contrasting shade tolerance change rank during ontogeny. Funct Ecol 18:820–828
Lusk CH, Reich PB (2000) Relationships of leaf dark respiration with light environment and tissue nitrogen content in juveniles of 11 cold-temperate tree species. Oecologia 123:318–329
Lusk CH, Chazdon RL, Hofmann G (2006) A bounded null model explains juvenile tree community structure along light availability gradients in a temperate rain forest. Oikos 112:131–137
Lusk CH, Falster DS, Jara-Vergara CK, Jimenez-Castillo M, Saldaña A (2008) Ontogenetic variation in light requirements of juvenile rainforest evergreens. Funct Ecol 22:454–459
Lusk CH, Onoda Y, Kooyman R, Gutiérrez-Girón A (2010) Reconciling species-level vs. plastic responses of evergreen leaf structure to light gradients: shade leaves punch above their weight. New Phytol 186:429–438
Mascaro J, Schnitzer SA, Carson WP (2004) Liana diversity, abundance and mortality in a tropical wet forest in Costa Rica. For Ecol Manag 190:3–14
Matesanz S, Gianoli E, Valladares F (2010) Global change and the evolution of phenotypic plasticity in plants. Ann NY Acad Sci 1206:35–55
Mitchell-Olds T, Shaw RG (1987) Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41:1149–1161
Montgomery RA, Chazdon RL (2002) Light gradient partitioning by tropical tree seedlings in the absence of canopy gaps. Oecologia 131:165–174
Nabe-Nielsen J (2002) Growth and mortality rates of the liana Machaerium cuspidatum in relation to light and topographic position. Biotropica 34:319–322
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. Johns Hopkins University Press, Baltimore
Poorter L (2009) Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New Phytol 181:890–900
Putz FE (1984) The natural history of lianas on Barro Colorado Island, Panama. Ecology 65:1713–1724
Rausher MD (1992) The measurement of selection on quantitative traits: biases due to environmental covariances between traits and fitness. Evolution 46:616–626
Ray TS (1992) Foraging behaviour in tropical herbaceous climbers (Araceae). J Ecol 80:189–203
Reich PB, Tjoelker MG, Walters MB, Vanderklein DW, Buschena C (1998) Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Funct Ecol 12:327–338
Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164:S143–S164
Rich PM (1990) Characterizing plant canopies with hemispherical photographs. Rem Sens Rev 5:13–29
Richardson AD, Duigan SP, Berlyn GP (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153:185–194
Riveros M, Humaña AM, Arroyo MTK (1996) Sistemas de reproducción en especies del bosque valdiviano (40° Latitud Sur). Phyton 58:167–176
Rodríguez R, Marticorena C (1987) Las especies del genero Luzuriaga R. et P. Gayana Bot 44:3–15
Saldaña A, Lusk CH (2003) Influence of overstorey species identity on resource availability and variation in composition of advanced regeneration in a temperate rainforest in southern Chile. Rev Chil Hist Nat 76:639–650
Saldaña A, Gianoli E, Lusk CH (2005) Ecophysiological responses to light availability in three Blechnum species (Pteridophyta, Blechnaceae) of different ecological breadth. Oecologia 145:252–257
Saldaña A, Lusk CH, Gonzáles WL, Gianoli E (2007) Natural selection on ecophysiological traits of a fern species in a temperate rainforest. Evol Ecol 21:651–662
Salgado-Luarte C, Gianoli E (2011) Herbivory may modify functional responses to shade in seedlings of a light-demanding tree species. Funct Ecol 25:492–499
Salgado-Luarte C, Gianoli E (2012) Herbivores modify selection on plant functional traits in a temperate rainforest understory. Am Nat 180:E42–E53
Schnitzer SA, Bongers F (2002) The ecology of lianas and their role in forests. Trends Ecol Evol 17:223–230
Sims DA, Gebauer RLE, Pearcy RW (1994) Scaling sun and shade photosynthetic acclimation of Alocasia macrorrhiza to whole-plant performance. 2. Simulation of carbon balance and growth at different photon flux densities. Plant Cell Environ 17:889–900
Smith-Ramírez C, Armesto JJ (1994) Flowering and fruiting patterns in the temperate rainforest of Chiloé, Chile—ecologies and climatic constraints. J Ecol 82:353–365
Stevens PF (2011) Angiosperm phylogeny website. Version 11 (WWW document). http://www.mobot.org/MOBOT/research/APweb/. Accessed 22 May 2011
Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 39:237–257
Valladares F, Gianoli E, Gómez JM (2007) Ecological limits to plant phenotypic plasticity. New Phytol 176:749–763
Valladares F, Gianoli E, Saldaña A (2011a) Climbing plants in a temperate rainforest understorey: searching for high light or coping with deep shade? Ann Bot 108:231–239
Valladares F, Saldaña A, Gianoli E (2011b) Costs versus risks: architectural changes with changing light quantity and quality in saplings of temperate rainforest trees of different shade tolerance. Aust Ecol 37:35–43
Walters MB, Reich PB (1996) Are shade tolerance, survival, and growth linked? low light and nitrogen effects on hardwood seedlings. Ecology 77:841–853
Walters MB, Reich PB (1999) Low-light carbon balance and shade tolerance in the seedlings of woody plants: do winter deciduous and broad-leaved evergreen species differ? New Phytol 143:143–154
Weinig C (2000) Differing selection in alternative competitive environments: shade-avoidance responses and germination timing. Evolution 54:124–136
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14
Acknowledgments
We are grateful to Fernando Carrasco for his valuable help in the field. We thank Brooke Jacobs, Cristian Salgado and Rodrigo Ríos for thoughtful comments that improved earlier versions of the manuscript. We thank CONAF (National Forestry Corporation) for granting permits to work in Puyehue National Park. All research activities complied with Chilean law. This study was funded by FONDECYT grant 1070503. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fernando Valladares.
Rights and permissions
About this article
Cite this article
Gianoli, E., Saldaña, A. Phenotypic selection on leaf functional traits of two congeneric species in a temperate rainforest is consistent with their shade tolerance. Oecologia 173, 13–21 (2013). https://doi.org/10.1007/s00442-013-2590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2590-2