Abstract
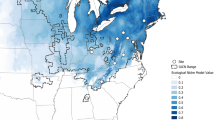
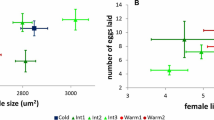
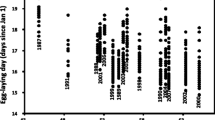
Little is known about intraspecific variation in fitness performance in response to thermal stress among natural populations and how this relates to evolutionary aspects of species ecology. In this study, population growth rate (PGR; a composite fitness measure) varied among five natural Chironomus riparius populations sampled across a climatic gradient when subjected to three temperature treatments reflecting the typical range of summer habitat temperatures (20, 24 and 28 °C). The variation could be explained by a complex model including effects of genetic drift, genetic diversity and adaptation to average temperature during the warmest month, in addition to experimental temperature. All populations suffered a decrease in PGR from 20 to 28 °C and ΔPGR was significantly correlated with the respective average habitat temperature in the warmest month—populations from warmer areas showing lower ΔPGR. This implies that long-term exposure to higher temperatures in the warmest month (the key reproductive period for C. riparius) is likely to be a key selective force influencing fitness at higher temperatures. A comparison of phenotypic divergence and neutral genetic differentiation revealed that one phenotypic trait—the number of fertile egg masses per female—appeared to be under positive selection in some populations. Our findings support a role for response to temperature selection along a climatic gradient and suggest population history is a key determinant of intraspecific fitness variation. We stress the importance of integrating different types of data (climatic, experimental, genetic) in order to understand the effects of global climate change on biodiversity.




Similar content being viewed by others
References
Armitage PD, Cranston PS, Pinder LCV (1995) The Chironomidae: biology and ecology of non-biting midges. Chapman & Hall, London
Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F (1996–2004) GENETIX 4.05, logiciel sous Windows TM pour la génétique des populations. In: Laboratoire génome, populations, interactions. CNRS UMR 5000, Université de Montpellier II, Montpellier
Bohonak AJ (1999) Dispersal, gene flow, and population structure. Q Rev Biol 74:21–45. doi:10.1086/392950
Brommer JE (2011) Whither P-st? The approximation of Q(st) by P-st in evolutionary and conservation biology. J Evol Biol 24:1160–1168. doi:10.1111/j.1420-9101.2011.02268.x
Coulson T, Kruuk LEB, Tavecchia G, Pemberton JM, Clutton-Brock TH (2003) Estimating selection on neonatal traits in red deer using elasticity path analysis. Evolution 57:2879–2892. doi:10.1554/03-126
Deutsch CA, et al. (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672. doi:10.1073/pnas.0709472105
Folguera G, Bastias DA, Bozinovic F (2009) Impact of experimental thermal amplitude on ectotherm performance: adaptation to climate change variability? Comp Biochem Physiol A Mol Integr Physiol 154:389–393. doi:10.1016/j.cbpa.2009.07.008
Frankham R (2005) Stress and adaptation in conservation genetics. J Evol Biol 18:750–755. doi:10.1111/j.1420-9101.2005.00885.x
Frouz J, Ali A, Lobinske RJ (2002) Influence of temperature on developmental rate, wing length, and larval head capsule size of pestiferous midge Chironomus crassicaudatus (Diptera: Chironomidae). J Econ Entomol 95:699–705. doi:10.1603/0022-0493-95.4.699
Hoffmann AA, Sgro CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485. doi:10.1038/nature09670
Hommen U (2005) Ableitung von Populationswachstumsraten aus Lebensdatenstudien mit Chironomus riparius. Frauenhofer Institut für Molekularbiologie und angewandte Ökologie, Schmallenberg
Huey RB, Kingsolver JG (1989) Evolution of thermal sensitivity of ectotherm performance. Trends Ecol Evol 4:131–135. doi:10.1016/0169-5347(89)90211-5
Huey RB, Kingsolver JG (1993) Evolution of resistance to high-temperature in ectotherms. Am Nat 142:S21–S46. doi:10.1086/285521
Huey RB, Partridge L, Fowler K (1991) Thermal sensitivity of Drosophila melanogaster responds rapidly to laboratory natural selection. Evolution 45:751–756. doi:10.2307/2409925
Kingsolver JG, Gomulkiewicz R (2003) Environmental variation and selection on performance curves. Integr Comp Biol 43:470–477. doi:10.1093/icb/43.3.470
Le Rouzic A, Carlborg O (2008) Evolutionary potential of hidden genetic variation. Trends Ecol Evol 23:33–37. doi:10.1016/j.tree.2007.09.014
Liefting M, Hoffmann AA, Ellers J (2009) Plasticity versus environmental canalization: population differences in thermal responses along a latitudinal gradient in Drosophila serrata. Evolution 63:1954–1963. doi:10.1111/j.1558-5646.2009.00683.x
Lynch M (2007) The origins of genome architecture, 1st edn. Sinauer, Massachusetts
MacIsaac HJ, Hebert PDN, Schwartz SS (1985) Inter- and intraspecific variation in acute thermal tolerance of Daphnia. Physiol Zool 58:350–355
Miehlbradt J, Neumann D (1976) Reproductive isolation via optical swarming behavior in sympatric Chironomus thimmi and Chironomus piger. Behaviour 58:272–297. doi:10.1163/156853976x00190
Nemec S, Heß M, Nowak C, Pfenninger M (2012) Experimental evidence for niche segregation in a sister species pair of non-biting midges. Hydrobiologia 691:203–212. doi:10.1007/s10750-012-1074-4
Nowak C, Hankeln T, Schmidt ER, Schwenk K (2006) Development and localization of microsatellite markers for the sibling species Chironomus riparius and Chironomus piger (Diptera: Chironomidae). Mol Ecol Notes 6:915–917. doi:10.1111/j.1471-8286.2006.01398.x
Nowak C, Vogt C, Diogo JB, Schwenk K (2007) Genetic impoverishment in laboratory cultures of the test organism Chironomus riparius. Environ Toxicol Chem 26:1018–1022. doi:10.1897/06-349r.1
Nowak C, Czeikowitz A, Vogt C, Oetken M, Streit B, Schwenk K (2008) Variation in sensitivity to cadmium among genetically characterized laboratory strains of the midge Chironomus riparius. Chemosphere 71:1950–1956. doi:10.1016/j.chemosphere.2007.12.023
OECD (2004) Sediment-water chironomid toxicity test using spiked water. OECD guidelines for the testing of chemicals (original guideline 219, adopted 13 April 2004)
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. doi:10.1111/j.1471-8286.2005.01155.x
Pinder LCV (1986) Biology of freshwater Chironomidae. Annu Rev Entomol 31:1–23
Reed DH, Frankham R (2001) How closely correlated are molecular and quantitative measures of genetic variation? A meta-analysis. Evolution 55:1095–1103. doi:10.1554/0014-3820(2001)055
Salamin N, Wueest RO, Lavergne S, Thuiller W, Pearman PB (2010) Assessing rapid evolution in a changing environment. Trends Ecol Evol 25:692–698. doi:10.1016/j.tree.2010.09.000
Sandrock C, Razmjou J, Vorburger C (2011) Climate effects on life cycle variation and population genetic architecture of the black bean aphid, Aphis fabae. Mol Ecol 20:4165–4181. doi:10.1111/j.1365-294X.2011.05242.x
Sankarperumal G, Pandian TJ (1991) Effect of temperature and chlorella density on growth and metamorphosis of Chironomus circumdatus (Kieffer) (Diptera). Aquat Insects 13:167–177. doi:10.1080/01650429109361438
Sibly RM, Hone J (2002) Population growth rate and its determinants: an overview. Phil Trans R Soc Lond Ser B Biol Sci 357:1153–1170. doi:10.1098/rstb.2002.1117
Stefan HG, Preudhomme EB (1993) Stream temperature estimation from air temperature. Water Resour Bull 29:27–45
Stevens MM (1998) Development and survival of Chironomus tepperi Skuse (Diptera: Chironomidae) at a range of constant temperatures. Aquat Insects 20:181–188
Urbanski J, Mogi M, O’Donnell D, DeCotiis M, Toma T, Armbruster P (2012) Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am Nat 179:490–500. doi:10.1086/664709
Vogt C, Belz D, Galluba S, Nowak C, Oetken M, Oehlmann J (2007a) Effects of cadmium and tributyltin on development and reproduction of the non-biting midge Chironomus riparius (Diptera)—baseline experiments for future multi-generation studies. J Environ Sci Health Part A-Toxic/Hazard Subst Environ Eng 42:1–9. doi:10.1080/10934520601015255
Vogt C, et al. (2007b) Interaction between genetic diversity and temperature stress on life-cycle parameters and genetic variability in midge Chironomus riparius populations. Clim Res 33:207–214. doi:10.3354/cr033207
Webb BW, Clack PD, Walling DE (2003) Water–air temperature relationships in a Devon river system and the role of flow. Hydrol Process 17:3069–3084. doi:10.1002/hyp.1280
Whitlock MC (2008) Evolutionary inference from QST. Mol Ecol 17:1885–1896. doi:10.1111/j.1365-294X.2008.03712.x
Winne CT, Keck MB (2005) Intraspecific differences in thermal tolerance of the diamondback water snake (Nerodia rhombifer): effects of ontogeny, latitude, and sex. Comp Biochem Physiol A Mol Integr Physiol 140:141–149. doi:10.1016/j.cbpb.2004.11.009
Winnepenninckx B, Backeljau T, Dewachter R (1993) Extraction of high-molecular-weight DNA from mollusks. Trends Genet 9:407
Acknowledgements
This work was supported by the research funding programme LOEWE (Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz) of the Hesse Ministry of Higher Education, Research, and the Arts. We appreciate the assistance of Miriam Imo and Joao Barateiro Diogo in field sampling and experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carla Caceres.
Rights and permissions
About this article
Cite this article
Nemec, S., Patel, S., Nowak, C. et al. Evolutionary determinants of population differences in population growth rate × habitat temperature interactions in Chironomus riparius . Oecologia 172, 585–594 (2013). https://doi.org/10.1007/s00442-012-2517-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2517-3