Abstract
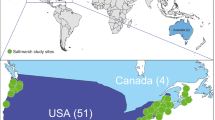
We examined geographic variation in the structure and function of salt marsh communities along the Atlantic and Gulf coasts of the United States. Focusing on the arthropod community in the dominant salt marsh plant Spartina alterniflora, we tested two hypotheses: first, that marsh community structure varies geographically, and second, that two aspects of marsh function (response to eutrophication and addition of dead plant material) also vary geographically. We worked at eleven sites on the Gulf Coast and eleven sites on the Atlantic Coast, dividing each coast up into two geographic areas. Abiotic conditions (tidal range, soil organic content, and water content, but not soil salinity), plant variables (Spartina nitrogen content, height, cover of dead plant material, but not live Spartina percent cover or light interception), and arthropod variables (proportional abundances of predators, sucking herbivores, stem-boring herbivores, parasitoids, and detritivores, but not total arthropod numbers) varied among the four geographic regions. Latitude and mean tidal range explained much of this geographic variation. Nutrient enrichment increased all arthropod functional groups in the community, consistent with previous experimental results, and had similar effects in all geographic regions, contrary to our hypothesis, suggesting widespread consistency in this aspect of ecosystem function. The addition of dead plant material had surprisingly little effect on the arthropod community. Our results caution against the uncritical extrapolation of work done in one geographic region to another, but indicate that some aspects of marsh function may operate in similar ways in different geographic regions, despite spatial variation in community structure.





Similar content being viewed by others
References
Aerts R (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. J Ecol 94:713–724
Anderson CM, Treshow M (1980) A review of environmental and genetic factors that affect height in Spartina alterniflora Loisel (salt-marsh cordgrass). Estuaries 3:168–176
Bertness MD, Ewanchuk PJ (2002) Latitudinal and climate-driven variation in the strength and nature of biological interactions in New England salt marshes. Oecologia 132:392–401
Bertness MD, Pennings SC (2000) Spatial variation in process and pattern in salt marsh plant communities in eastern North America. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer, Dordrecht, pp 39–57
Bertness MD, Ewanchuk PJ, Silliman BR (2002) Anthropogenic modification of New England salt marsh landscapes. Proc Nat Acad Sci USA 99:1395–1398
Bertness MD, Crain C, Holdredge C, Sala N (2008) Eutrophication and consumer control of New England salt marsh primary productivity. Conserv Biol 22:131–139
Bowdish TI, Stiling P (1998) The influence of salt and nitrogen on herbivore abundance: direct and indirect effects. Oecologia 113:400–405
Brook AJ, Woodcock BA, Sinka M, Vanbergen AJ (2008) Experimental verification of suction sampler capture efficiency in grasslands of differing vegetation height and structure. J Appl Ecol 45:1357–1363
Costa CSB, Marangoni JC, Azevedo AMG (2003) Plant zonation in irregularly flooded salt marshes: relative importance of stress tolerance and biological interactions. J Ecol 91:951–965
Craft C (2007) Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S. tidal marshes. Limnol Oceanogr 52:1220–1230
Deegan LA et al (2007) Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecol Appl 17:S42–S63
Denno RE et al (1996) Habitat persistence underlies intraspecific variation in the dispersal strategies of planthoppers. Ecol Monogr 66:389–408
Denno RF, Gratton C, Peterson MA, Langellotto GA, Finke DL, Huberty AF (2002) Bottom-up forces mediate natural-enemy impact in a phytophagous insect community. Ecology 83:1443–1458
Denno RF, Gratton C, Dobel H, Finke DL (2003) Predation risk affects relative strength of top-down and bottom-up impacts on insect herbivores. Ecology 84:1032–1044
Denno RF, Lewis D, Gratton C (2005) Spatial variation in the relative strength of top-down and bottom-up forces: causes and consequences for phytophagous insect populations. Ann Zool Fenn 42:295–311
Dobel HG, Denno RF, Coddington JA (1990) Spider (Araneae) community structure in an intertidal salt-marsh—effects of vegetation structure and tidal flooding. Environ Entomol 19:1356–1370
Farina JM, Silliman BR, Bertness MD (2009) Can conservation biologists rely on established community structure rules to manage novel systems?... Not in salt marshes. Ecol Appl 19:413–422
Finke DL, Denno RF (2006) Spatial refuge from intraguild predation: implications for prey suppression and trophic cascades. Oecologia 149:265–275
Gaeta JW, Kornis MS (2010) Stem borer frequency and composition in healthy Spartina alterniflora (smooth cordgrass) and dieback zones in a southern Atlantic coast salt marsh. Estuaries Coasts 34:1078–1083
Gratton C, Denno RF (2003a) Inter-year carryover effects of a nutrient pulse on Spartina plants, herbivores, and natural enemies. Ecology 84:2692–2707
Gratton C, Denno RF (2003b) Seasonal shift from bottom-up to top-down impact in phytophagous insect populations. Oecologia 134:487–495
Gripenberg S, Roslin T (2007) Up or down in space? Uniting the bottom-up versus top-down paradigm and spatial ecology. Oikos 116:181–188
Herke SW, Foltz DW (2002) Phylogeography of two squid (Loligo pealei and L. plei) in the Gulf of Mexico and northwestern Atlantic Ocean. Mar Biol 140:103–115
Hines J, Megonigal JP, Denno RF (2006) Nutrient subsidies to belowground microbes impact aboveground food web interactions. Ecology 87:1542–1555
Huntington TG (2006) Available water capacity and soil organic matter. In: Lal R (ed) Encyclopedia of soil science, vol 1, 2nd edn. CRC, Boca Raton, pp 139–143
Kneib RT (1984) Patterns of invertebrate distribution and abundance in the intertidal salt-marsh—causes and questions. Estuaries 7:392–412
Kunza AE, Pennings SC (2008) Patterns of plant diversity in Georgia and Texas salt marshes. Estuaries Coasts 31:673–681
Langellotto GA, Denno RF (2006) Refuge from cannibalism in complex-structured habitats: implications for the accumulation of invertebrate predators. Ecol Entomol 31:575–581
Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967
Levine JM, Brewer JS, Bertness MD (1998) Nutrients, competition and plant zonation in a New England salt marsh. J Ecol 86:285–292
Lewis D, Denno RF (2009) A seasonal shift in habitat suitability enhances an annual predator subsidy. J Anim Ecol 78:752–760
McFarlin CR, Brewer JS, Buck TL, Pennings SC (2008) Impact of fertilization on a salt marsh food web in Georgia. Estuaries Coasts 31:313–325
McKee KL, Patrick WH (1988) The relationship of smooth cordgrass (Spartina alterniflora) to tidal datums—a review. Estuaries 11:143–151
Meade RH (1969) Landward transport of bottom sediments in estuaries of Atlantic coastal plain. J Sediment Petrol 39:222
Mitsch WJ, Gosselink JG (1993) Wetlands, 2nd edn. Van Nostrand Reinhold, New York
Moon DC, Moon JC (2010) Effects of environmental stress cascade up through four trophic levels in a salt marsh study system. Ecol Entomol 35:721–726
NOAA (2011) NOAA Tides & Currents Online Database, vols. 2009, 2010. National Oceanic and Atmospheric Administration, Silver Spring
Odum WE (1988) Comparative ecology of tidal fresh-water and salt marshes. Annu Rev Ecol Syst 19:147–176
Pennings SC, Bertness MD (2001) Salt marsh communities. In: Bertness MD, Gaines SD, Hay M (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 289–316
Pennings SC, Stanton LE, Brewer JS (2002) Nutrient effects on the composition of salt marsh plant communities along the southern Atlantic and Gulf coasts of the United States. Estuaries 25:1164–1173
Pennings SC, Selig ER, Houser LT, Bertness MD (2003) Geographic variation in positive and negative interactions among salt marsh plants. Ecology 84:1527–1538
Pennings SC et al (2005) Do individual plant species show predictable responses to nitrogen addition across multiple experiments? Oikos 110:547–555
Pennings SC et al (2009) Latitudinal variation in herbivore pressure in Atlantic Coast salt marshes. Ecology 90:183–195
Randlkofer B, Obermaier E, Casas J, Meiners T (2010) Connectivity counts: disentangling effects of vegetation structure elements on the searching movement of a parasitoid. Ecol Entomol 35:446–455
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Nat Acad Sci USA 101:11001–11006
Sala NM, Bertness MD, Silliman BR (2008) The dynamics of bottom-up and top-down control in a New England salt marsh. Oikos 117:1050–1056
Siemann E (1998) Experimental tests of effects of plant productivity and diversity on grassland arthropod diversity. Ecology 79:2057–2070
Siska EL, Pennings SC, Buck TL, Hanisak MD (2002) Latitudinal variation in palatability of salt-marsh plants: which traits are responsible? Ecology 83:3369–3381
Stiling PD, Strong DR (1983) Weak competition among Spartina stem borers, by means of murder. Ecology 64:770–778
Turner RE (1976) Geographic variations in salt marsh macrophyte production: a review. Contrib Mar Sci 20:47–68
Valiela I, Teal JM (1979) Nitrogen budget of a salt marsh ecosystem. Nature 280:652–656
Valiela I, Teal JM, Sass WJ (1975) Production and dynamics of salt-marsh vegetation and effects of experimental treatment with sewage sludge—biomass, production, and species composition. J Appl Ecol 12:973–981
White TCR (1984) The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63:90–105
Williams DA, Brown SD, Crawford DL (2008) Contemporary and historical influences on the genetic structure of the estuarine-dependent Gulf killifish Fundulus grandis. Marine Ecol Prog Ser 373:111–121
Wimp GM, Murphy SM, Finke DL, Huberty AF, Denno RF (2010) Increased primary production shifts the structure and composition of a terrestrial arthropod community. Ecology 91:3303–3311
Zhang DQ, Hui DF, Luo YQ, Zhou GY (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93
Acknowledgments
We thank T. Decker, B. DeLong, C. Gratton, C. Grimm, H. Guo, C-K. Ho, L. Marczak, J. Martinez, M. Richardson, A. Stark, K. Wieski, G. Wimp, and H. Vu for help in the field and laboratory, and K. Wieski for help with the SEM analysis. We thank B. Cole, A. Frankino, and E. Siemann as well as two anonymous reviewers for advice and comments on the manuscript. We are grateful to the sponsors and staff of the 22 field sites where we worked for facilitating access and welcoming our activities. We thank the National Science Foundation (DEB-0638796, OCE-1045221, OCE-0620959) for financial support. This is contribution number 1015 from the University of Georgia Marine Institute. This work is a contribution of the Georgia Coastal Ecosystems Long-Term Ecological Research program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Pete Peterson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McCall, B.D., Pennings, S.C. Geographic variation in salt marsh structure and function. Oecologia 170, 777–787 (2012). https://doi.org/10.1007/s00442-012-2352-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2352-6