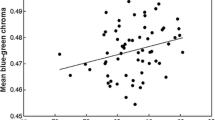
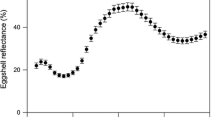
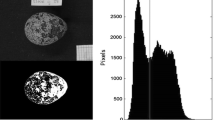
Abstract
Many passerine species lay eggs that are speckled with dark protoporphyrin pigmentation. Because protoporphyrin is mainly derived from the blood, we here formulate and test a new hypothesis that links an increase in anaemia along the laying sequence to within-clutch variation in egg pigmentation. More intense pigmentation is expected if pigments accumulate during enhanced red blood cell production in response to anaemia. Reduced pigmentation is expected if pigments are derived from the degradation of red blood cells that circulate in smaller numbers due to blood loss. To test this hypothesis, we manipulated anaemia in great tit (Parus major) females by infesting the nests with hen fleas (Ceratophyllus gallinae) prior to egg laying. Polychromatophil (i.e., immature red blood cells) percentage, as a measure of blood cell production, was positively correlated with parasite load confirming that female great tits experienced stronger anaemia when infested with haematophagous parasites during egg laying. We found a positive relationship between spot darkness and laying order that weakened under high parasite load. This result suggests that anaemia in females due to blood-sucking parasites led to diminished protoporphyrin from disintegrated red blood cells and hence a decreased deposition of protoporphyrin. However, the overall increase in pigment darkness along the laying sequence suggests that pigments also accumulate by enhanced red blood cell production caused by anaemia due to egg production itself.

Similar content being viewed by others
References
Baird T, Solomon SE, Tedstone DR (1975) Localization and characterization of egg shell porphyrins in several avian species. Br Poult Sci 16:201–208
Campbell TW, Ellis CK (2007) Avian and exotic animal hematology and cytology, 3rd edn. Blackwell, Oxford
Challenger WO, Williams TD, Christians JK, Vezina F (2001) Follicular development and plasma yolk precursor dynamics through the laying cycle in the European starling (Sturnus vulgaris). Physiol Biochem Zool 74:356–365. doi:10.1086/320427
Cherry MI, Bennett ATD, Moskat C (2007) Do cuckoos choose nests of great reed warblers on the basis of host egg appearance? J Evol Biol 20:1218–1222. doi:10.1111/j.1420-9101.2007.01308.x
Christe P, Richner H, Oppliger A (1996) Of great tits and fleas: sleep baby sleep. Anim Behav 52:1087–1092
De Coster G, De Neve L, Martín-Gálvez D, Therry L, Lens L (2010) Variation in innate immunity in relation to ectoparasite load, age and season: a field experiment in great tits (Parus major). J Exp Biol 213:3012–3018. doi:10.1242/jeb.042721
Fernandez FR, Grindem CB (2006) Reticulocyte response. In: Feldman BF, Zinkl JG, Jain NC (eds) Schalm’s veterinary hematology, 5th edn. Blackwell, Ames
Fitze PS, Tschirren B, Richner H (2004) Life history and fitness consequences of ectoparasites. J Anim Ecol 73:216–226
Gosler AG, Barnett PR, Reynolds SJ (2000) Inheritance and variation in eggshell patterning in the great tit Parus major. Proc R Soc Lond B 267:2469–2473
Gosler AG, Higham JP, Reynolds SJ (2005) Why are birds’ eggs speckled? Ecol Lett 8:1105–1113
Harper GH, Marchant A, Boddington DG (1992) The ecology of the hen flea Ceratophyllus gallinae and the moorhen flea Dasypsyllus gallinulae in nestboxes. J Anim Ecol 61:317–327
Heeb P, Werner I, Richner H, Kölliker M (1996) Horizontal transmission and reproductive rates of hen fleas in great tit nests. J Anim Ecol 65:474–484
Higham JP, Gosler AG (2006) Speckled eggs: water-loss and incubation behaviour in the great tit Parus major. Oecologia 149:561–570. doi:10.1007/s00442-006-0484-2
Jagannath A, Shore RF, Walker LA, Ferns PN, Gosler AG (2008) Eggshell pigmentation indicates pesticide contamination. J Appl Ecol 45:133–140. doi:10.1111/j.1365-2664.2007.01386.x
Johns JL, Shooshtari MP, Christopher MM (2008) Development of a technique for quantification of reticulocytes and assessment of erythrocyte regenerative capacity in birds. Am J Vet Res 69:1067–1072
Jones PJ (1983) Haematocrit values of breeding Red-billed queleas Quelea quelea (Aves:Ploceidae) in relation to body condition and thymus activity. J Zool 201:217–222
Kennedy GY, Vevers HG (1973) Eggshell pigments of Araucano fowl. Comp Biochem Physiol 44:11–25
Kennedy GY, Vevers HG (1976) Survey of avian eggshell pigments. Comp Biochem Physiol B Biochem Mol Biol 55:117–123
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Kern MD, De Graw WA, King JR (1972) Effects of gonadal hormones on the blood composition of white-crowned sparrows. Gen Comp Endocrinol 18:43–53
Langer EE, Labbe RF, Crosby EF, Haining RG, Jacobs P, Finch CA (1972) Erythrocyte protoporphyrin. Blood 40:112–128
López de Hierro M, De Neve L (2010) Pigment limitation and female reproductive characteristics influence egg shell spottiness and ground colour variation in the house sparrow (Passer domesticus). J Ornithol 151:833–840. doi:10.1007/s10336-010-0520-1
López-Rull I, Gil M, Gil D (2007) Spots in starling Sturnus unicolor eggs are good indicators of ectoparasite load by Carnus hemapterus (Diptera:Carnidae). Ardeola 54:131–134
Mahler B, Confalonieri VA, Lovette IJ, Reboreda JC (2008) Eggshell spotting in brood parasitic shiny cowbirds (Molothrus bonariensis) is not linked to the female sex chromosome. Behav Ecol Sociobiol 62:1193–1199. doi:10.1007/s00265-008-0548-x
Martínez-de la Puente J et al (2007) Are eggshell spottiness and colour indicators of health and condition in blue tits Cyanistes caeruleus? J Avian Biol 38:377–384. doi:10.1111/j.2007.0908-8857.03877.x
Martínez-Padilla J, Dixon H, Vergara P, Pérez-Rodríguez L, Fargallo JA (2010) Does egg colouration reflect male condition in birds? Naturwissenschaften 97:469–477. doi:10.1007/s00114-010-0660-4
Merino S et al (1998) Increase in a heat-shock protein from blood cells in response of nestling house martins (Delichon urbica) to parasitism: an experimental approach. Oecologia 116:343–347
Merino S, Martínez J, Møller AP, Barbosa A, De Lope F, Rodríguez-Caabeiro F (2002) Blood stress protein levels in relation to sex and parasitism of barn swallows (Hirundo rustica). Ecoscience 9:300–305
Miksik I, Holan V, Deyl Z (1994) Quantification and variability of eggshell pigment content. Comp Biochem Physiol A Physiol 109:769–772
Miksik I, Holan V, Deyl Z (1996) Avian eggshell pigments and their variability. Comp Biochem Physiol B Biochem Mol Biol 113:607–612
Moreno J, Osorno JL (2003) Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806. doi:10.1046/j.1461-0248.2003.00505.x
Moreno J, Morales J, Lobato E, Merino S, Tomás G, Martinez-de la Puente J (2005) Evidence for the signaling function of egg color in the pied flycatcher Ficedula hypoleuca. Behav Ecol 16:931–937. doi:10.1093/beheco/ari072
Pacejka AJ, Gratton CM, Thompson CF (1998) Potentially virulent mites affect house wren (Troglodytes aedon) reproductive success? Ecology 79:1797–1806
Reynolds SJ, Waldron S (1999) Body water dynamics at the onset of egg-laying in the zebra finch Taeniopygia guttata. J Avian Biol 30:1–6. doi:10.2307/3677236
Richner H, Oppliger A, Christe P (1993) Effect of an ectoparasite on reproduction in great tits. J Anim Ecol 62:703–710
Romanoff AL, Romanoff AJ (1949) The avian egg. Wiley, New York
Sanz JJ, García-Navas V (2009) Eggshell pigmentation pattern in relation to breeding performance of blue tits Cyanistes caeruleus. J Anim Ecol 78:31–41
Schielzeth H, Forstmeier W (2009) Conclusions beyond support: overconfident estimates in mixed models. Behav Ecol 20:416–420. doi:10.1093/beheco/arn145
Schwartz S, Wikoff HM (1952) The relation of erythrocyte coproporphyrin and protoporphyrin to erythropoiesis. J Biol Chem 194:563–573
Szabo K, Szalmas A, Liker A, Barta Z (2002) Effects of haematophagous mites on nestling house sparrows (Passer domesticus). Acta Parasitol 47:318–322
Tomás G, Merino S, Martínez J, Moreno J, Sanz JJ (2005) Stress protein levels and blood parasite infection in blue tits (Parus caeruleus): a medication field experiment. Ann Zool Fenn 42:45–56
Underwood TJ, Sealy SG (2002) Adaptative significance of egg coloration. In: Deeming DC (ed) Avian incubation. Behaviour, environment and evolution. Oxford University Press, Oxford
Verbeke G, Molenberghs G (2000) Linear mixed models for longitudinal data. Springer, New York
Wagner EC, Prevolsek JS, Wynne-Edwards KE, Williams TD (2008a) Hematological changes associated with egg production: estrogen dependence and repeatability. J Exp Biol 211:400–408. doi:10.1242/jeb.011205
Wagner EC, Stables CA, Williams TD (2008b) Hematological changes associated with egg production: direct evidence for changes in erythropoiesis but a lack of resource dependence? J Exp Biol 211:2960–2968. doi:10.1242/jeb.017897
Wang X-T et al (2007) Study of the deposition process of eggshell pigments using an improved dissolution method. Poult Sci 86:2236–2238
Wang XT et al (2009) Comparison of the total amount of eggshell pigments in Dongxiang brown-shelled eggs and Dongxiang blue-shelled eggs. Poult Sci 88:1735–1739. doi:10.3382/ps.2008-00434
Watson CJ (1935) Concerning the naturally occurring porphyrins. III. The isolation of coproporphyrin I from the feces of untreated cases of pernicious anemia. J Clin Invest 14:116–118
Williams TD, Challenger WO, Christians JK, Evanson M, Love O, Vézina F (2004) What causes the decrease in haematocrit during egg production? Funct Ecol 18:330–336
Zhao R, Xu G, Liu Z, Li J, Yang N (2006) A study on eggshell pigmentation: biliverdin in blue-shelled chickens. Poult Sci 85:546–549
Acknowledgments
We are grateful to A. d’Ursel and A. Beck for allowing us access to the forest, H. Matheve for help during fieldwork, D. Vercayie and F. Philips for scoring egg pigmentation, and Angelica Alcántara-Exposito for blood cell counts. We also thank D. Hanley and an anonymous reviewer for the most valuable comments that improved our paper considerably. This study was carried out with permission from the Animal Ethics Committee of Ghent University (ECP 08/05). G.D.C. was financially supported by a doctoral grant and by FWO research community WO.037.10 N from the Research Foundation Flanders (FWO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Oliver Love.
Rights and permissions
About this article
Cite this article
De Coster, G., De Neve, L. & Lens, L. Intraclutch variation in avian eggshell pigmentation: the anaemia hypothesis. Oecologia 170, 297–304 (2012). https://doi.org/10.1007/s00442-012-2304-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2304-1