Abstract
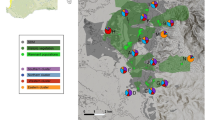
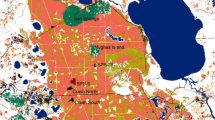
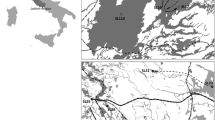
Local populations are subject to recurrent extinctions, and small populations are particularly prone to extinction. Both demographic (stochasticity and the Allee effect) and genetic factors (drift load and inbreeding depression) potentially affect extinction. In fragmented populations, regular dispersal may boost population sizes (demographic rescue effect) or/and reduce the local inbreeding level and genetic drift (genetic rescue effect), which can affect extinction risks. We studied extinction processes in highly fragmented populations of the common species Crepis sancta (Asteraceae) in urban habitats exhibiting a rapid turnover of patches. A four-year demographic monitoring survey and microsatellite genotyping of individuals allowed us to study the determinants of extinction. We documented a low genetic structure and an absence of inbreeding (estimated by multilocus heterozygosity), which suggest that genetic factors were not a major cause of patch extinction. On the contrary, local population size was the main factor in extinction, whereas connectivity was shown to decrease patch extinction, which we interpreted as a demographic rescue effect that was likely due to better pollination services for reproduction. This coupling of demographic and genetic tools highlighted the importance of dispersal in local patch extinctions of small fragmented populations connected by gene flow.



Similar content being viewed by others
References
Armbruster P, Reed DH (2005) Inbreeding depression in benign and stressful environments. Heredity 95(3):235–242
Belovsky GE, Mellison C, Larson C, Van Zandt PA (1999) Experimental studies of extinction dynamics. Science 286(5442):1175–1177
Berec L, Angulo E, Courchamp F (2007) Multiple Allee effects and population management. Trends Ecol Evol 22:185–191
Bijlsma R, Bundgaard J, Boerema AC (2000) Does inbreeding affect the extinction risk of small populations? Predictions from Drosophila. J Evol Biol 13(3):502–514
Brown JH, Kodric-Brown A (1977) Turnover rates in insular biogeography—effect of immigration on extinction. Ecology 58(2):445–449
Burnham K, Anderson D (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Chapuis MP, Estoup A (2007) Microsatellite null alleles and estimation of population differentiation. Mol Biol Evol 24(3):621–631
Cheptou PO, Avendano LG (2006) Pollination processes and the Allee effect in highly fragmented populations: consequences for the mating system in urban environments. New Phytol 172(4):774–783
Cheptou PO, Berger A, Blanchard A, Collin C, Escarre J (2000) The effect of drought stress on inbreeding depression in four populations of the Mediterranean outcrossing plant Crepis sancta (Asteraceae). Heredity 85(3):294–302
Cheptou PO, Lepart J, Escarre J (2002) Mating system variation along a successional gradient in the allogamous and colonizing plant Crepis sancta (Asteraceae). J Evol Biol 15(5):753–762
Cheptou PO, Carrue O, Rouifed S, Cantarel A (2008) Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proc Natl Acad Sci USA 105(10):3796–3799
Crow JF, Kimura M (1970) An introduction to population genetics. Harper and Row, New York
David P, Pujol B, Viard F, Castella V, Goudet J (2007) Reliable selfing rate estimates from imperfect population genetic data. Mol Ecol 16(12):2474–2487
Dempster AP, Laird NM, Rubin DB (1977) Maximum likelihood from incomplete data via the EM algorithm. J Roy Stat Soc B 39:1–38
Dennis B (2002) Allee effects in stochastic populations. Oikos 96:389–401
Dornier A, Pons V, Cheptou PO (2011) Colonization and extinction dynamic of an annual plant metapopulation in an urban environment. Oikos 120:1240–1246
Dubois MP, Dornier A, Jarne P, Cheptou PO (2007) Nine polymorphic microsatellite markers in Crepis sancta (Asteraceae). Mol Ecol Notes 7(4) :681–683
Ebert D, Haag C, Kirkpatrick M, Riek M, Hottinger JW, Pajunen VI (2002) A selective advantage to immigrant genes in a Daphnia metapopulation. Science 295:485–488
Frankham R (2005) Genetics and extinction. Biol Conserv 126(2):131–140
Freckelton RP, Watkinson AR (2002) Large-scale spatial dynamics of plants: metapopulations, regional ensembles and patchy populations. J Ecol 90(3):419–434
Gaggiotti O, Hanski I (2004) Mechanisms of population extinction. In: Hanski I, Gaggiotti OE (eds) Ecology, genetics, and evolution of metapopulations. Academic, San Diego, pp 337–366
Griffen BD, Drake JM (2008) A review of extinction in experimental populations. J Anim Ecol 77(6):1274–1287
Groom M.J (1998) Allee effects limit population viability of an annual plant. Am Nat 151(6):487–496
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Herlihy CR, Eckert CG (2002) Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 416(6878):320–323
Higgins K, Lynch M (2001) Metapopulation extinction caused by mutation accumulation. Proc Natl Acad Sci USA 98(5):2928–2933
Ives AR, Whitlock MC (2002) Inbreeding and metapopulations. Science 295:454–455
Jaquiéry J, Guillaume F, Perrin N (2009) Predicting the deleterious effects of mutation load in fragmented populations. Conserv Biol 23:207–218
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17(5):230–241
Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman TL (2005) Pollen limitation of plant reproduction: pattern and process. Ann Rev Ecol Evol Syst 36:467–497
Lande R, Engern S, Saether BE (2003) Stochastic population dynamics in ecology and conservation. Oxford University Press, Oxford
Lennartsson T (2002) Extinction thresholds and disrupted plant–pollinator interactions in fragmented plant populations. Ecology 83(11):3060–3072
Levins R (1969) Some demographic and genetic consequences of environment heterogeneity for biological control. Bull Entomol Soc Am 15:237–240
Lowe WH, Allendorf FW (2010) What can genetics tell us about population connectivity? Mol Ecol 19(15):3038–3051
Lynch M, Coner J, Burger R (1995) Mutation accumulation and the extinction of small populations. Am Nat 146(4):489–518
Madsen T, Shine R, Olsson M, Wittzell H (1999) Restoration of an inbred adder population. Nature 402:34–35
Matthies D, Brauer I, Maibom W, Tscharntke T (2004) Population size and the risk of local extinction: empirical evidence from rare plants. Oikos 105(3):481–488
Melbourne BA, Hastings A (2008) Extinction risk depends strongly on factors contributing to stochasticity. Nature 454(7200):100–103
Menges ES (1990) Population viability for an endangered plant. Conserv Biol 4:52–62
Moilanen A (1999) Patch occupancy models of metapopulation dynamics: efficient parameter estimation using implicit statistical inference. Ecology 80(3):1031–1043
Moilanen A, Nieminen M (2002) Simple connectivity measures in spatial ecology. Ecology 83(4):1131–1145
Newman D, Pilson D (1997) Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchella. Evolution 51(2):354–362
Noel F, Porcher E, Moret J, Machon N (2006) Connectivity, habitat heterogeneity, and population persistence in Ranunculus nodiflorus, an endangered species in France. New Phytol 169(1):71–83
Paland S, Schmid B (2003) Population size and the nature of genetic load in Gentianella germanica. Evolution 57(10):2242–2251
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145(4):1219–1228
Rousset F (2008) Genepop’007: a complete re-implementation of the Genepop software for Windows and Linux. Mol Ecol Res 8(1):103–106
Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I (1998) Inbreeding and extinction in a butterfly metapopulation. Nature 392(6675):491–494
Slatkin M (1977) Gene flow and genetic drift in a species subject to frequent local extinctions. Theor Pop Biol 12(3):253–262
Stacey P, Johnson V, Taper M (1997) Migration within metapopulations: the impact upon local population dynamics. In: Hanski I, Gilpin ME (eds) Metapopulation biology: ecology, genetics, and evolution. Academic, San Diego, pp 267–291
Stephens PA, Sutherland WJ (1999) Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol Evol 14(10):401–405
Tallmon DA, Luikart G, Waples RS (2004) The alluring simplicity and complex reality of genetic rescue. Trends Ecol Evol 19(9):489–496
Wade MJ, Mccauley DE (1988) Extinction and recolonization: their effects on the genetic differentiation of local populations. Evolution 42(5):995–1005
Whitlock MC (2002) Selection, load and inbreeding depression in a large metapopulation. Genetics 160(3):1191–1202
Whitlock MC, Mccauley DE (1990) Some population genetic consequences of colony formation and extinction—genetic correlations within founding groups. Evolution 44(7):1717–1724
Whitlock MC, McCauley DE (1999) Indirect measures of gene flow and migration: F ST ≠ 1/(4Nm + 1). Heredity 82:117–125
Wright S (1969) Evolution and the genetics of populations, vol 2: theory of gene frequencies. Chicago University Press, Chicago
Acknowledgments
We thank H. Freville and three anonymous reviewers for their helpful comments on the various versions of the manuscript, as well as A. Winn for useful comments and for editing the manuscript. The TrameVerte programme (ANR-0.8-VILL-0003) provided financial support for this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Alice Winn.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dornier, A., Cheptou, PO. Determinants of extinction in fragmented plant populations: Crepis sancta (asteraceae) in urban environments. Oecologia 169, 703–712 (2012). https://doi.org/10.1007/s00442-011-2229-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2229-0