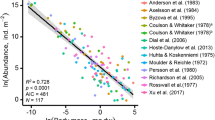
Abstract
Global environmental changes are expected to impact the abundance of plants and animals aboveground, but comparably little is known about the responses of belowground organisms. Using meta-analysis, we synthesized results from over 75 manipulative experiments in order to test for patterns in the effects of elevated CO2, warming, and altered precipitation on the abundance of soil biota related to taxonomy, body size, feeding habits, ecosystem type, local climate, treatment magnitude and duration, and greenhouse CO2 enrichment. We found that the positive effect size of elevated CO2 on the abundance of soil biota diminished with time, whereas the negative effect size of warming and positive effect size of precipitation intensified with time. Trophic group, body size, and experimental approaches best explained the responses of soil biota to elevated CO2, whereas local climate and ecosystem type best explained responses to warming and altered precipitation. The abundance of microflora and microfauna, and particularly detritivores, increased with elevated CO2, indicative of microbial C limitation under ambient CO2. However, the effects of CO2 were smaller in field studies than in greenhouse studies and were not significant for higher trophic levels. Effects of warming did not depend on taxon or body size, but reduced abundances were more likely to occur at the colder and drier sites. Precipitation limited all taxa and trophic groups, particularly in forest ecosystems. Our meta-analysis suggests that the responses of soil biota to global change are predictable and unique for each global change factor.

Similar content being viewed by others
References
Aerts R (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. J Ecol 94:713–724
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Allen AS, Schlesinger WH (2004) Nutrient limitations to soil microbial biomass and activity in loblolly pine forests. Soil Biol Biochem 36:581–589
Allison SD, Wallenstein MD, Bradford MA (2010) Soil–carbon response to warming dependent on microbial physiology. Nature Geosci 3:336–340
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Arnold SS, Fernandez IJ, Rustad LE, Zibilske LM (1999) Microbial response of an acid forest soil to experimental soil warming. Biol Fert Soils 30:239–244
Badeck FW, Bondeau A, Böttcher K, Doktor D, Lucht W, Schaber J, Sitch S (2004) Responses of spring phenology to climate change. New Phytol 162:295–309. doi:10.1111/j.1469-8137.2004.01059.x
Bakonyi G, Nagy P (2000) Temperature- and moisture-induced changes in the structure of the nematode fauna of a semiarid grassland-patterns and mechanisms. Glob Change Biol 6:697–707
Bardgett RD, Chan KF (1999) Experimental evidence that soil fauna enhance nutrient mineralization and plant nutrient uptake in montane grassland ecosystems. Soil Biol Biochem 31:1007–1014
Bardgett RD, Whittaker JB, Frankland JC (1993) The diet and food preferences of Onychiurus procampatus (Collembola) from upland grassland soils. Biol Fert Soils 16:296–298
Barker DH, Vanier C, Naumburg E, Charlet TN, Nielsen KM, Newingham BA, Smith SD (2006) Enhanced monsoon precipitation and nitrogen deposition affect leaf traits and photosynthesis differently in spring and summer in the desert shrub Larrea tridentate. New Phytol 169:799–808
Beare MH, Coleman DC, Crossley DA, Hendrix PF, Odum EP (1995) A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant Soil 170:5–22
Bokhorst S, Huikes A, Convey P, van Bodegom PM, Aerts R (2008) Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol Biochem 40:1547–1556
Bouwman LA, Zwart KB (1994) The ecology of bacterivorous protozoans and nematodes in arable soil. Agric Ecosyst Environ 51:145–160
Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE (2002a) Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 99:317–323
Bradford MA, Jones TH, Bardgett RD et al (2002b) Impacts of soil faunal community composition on model grassland ecosystems. Science 298:615–618
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11:1316–1327
Briones MJI, Ineson P, Sleep D (1999) Use of δ 13C to determine food selection in collembolan species. Soil Biol Biochem 31:937–940
Briones MJI, Ineson P, Heinemeyer A (2007) Predicting potential impacts of climate change on the geographical distribution of enchytraeids: a meta-analysis approach. Glob Change Biol 13:2252–2269
Briones MJI, Ostle NJ, McNamara NP, Poskitt J (2009) Functional shifts of grassland soil communities in response to soil warming. Soil Biol Biochem 41:315–322
Brussaard L (1998) Soil fauna, guilds, functional groups and ecosystem processes. Appl Soil Ecol 9:123–135
Brussaard L, Behan-Pelletier VM, Bignell DE et al (1997) Biodiversity and ecosystem functioning in soil. Ambio 26:563–570
Bushby HVA, Marshall KC (1977) Some factors affecting the survival of root-nodule bacteria on desiccation. Soil Biol Biochem 9:143–147
Chapin FS, Walker BH, Hobbs RJ, Hooper DU, Lawton JH, Sala OE, Tilman D (1997) Biotic control over the functioning of ecosystems. Science 277:500–504
Chen B, Snider RJ, Snider RM (1996) Food consumption by Collembola from northern Michigan deciduous forest. Pedobiologia 40:149–161
Clarholm M (1989) Effects of plant–bacterial–amoebal interactions on plant uptake of nitrogen under field conditions. Biol Fert Soils 8:373–378
Cole L, Bardgett RD, Ineson P, Adamson JK (2002) Relationships between enchytraeid worms (Oligochaeta), climate change, and the release of dissolved organic carbon from blanket peat in northern England. Soil Biol Biochem 34:599–607
Convey P, Wynn-Williams DD (2002) Antarctic soil nematode response to artificial climate amelioration. Eur J Soil Biol 38:255–259
Convey P, Pugh A, Jackson C, Murray AW, Ruhland CT, Xiong FS, Day TA (2002) Response of Antarctic terrestrial microarthropods to long-term climate manipulations. Ecology 83:3130–3140
Cornejo FH, Varela A, Wright SJ (1994) Tropical forest decomposition under seasonal drought: nutrient release, fungi and bacteria. Oikos 70:183–190
Coulson SJ, Hodkinson ID, Webb NR, Block W, Bale JS, Strathdee AT, Worland MR, Wooley C (1996) Effects of experimental temperature elevation on high-arctic soil microarthropod populations. Polar Biol 16:147–153
Coulson SJ, Leinaas HP, Ims RA, Søvik G (2000) Experimental manipulation of the winter surface ice layer: the effects on a High Arctic soil microarthropod community. Ecography 23:299–306
Cragg RG, Bardgett RD (2001) How changes in soil faunal diversity and composition within a trophic group influence decomposition processes. Soil Biol Biochem 33:2073–2081
De Graaff MA, van Groenigen KJ, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Change Biol 12:2077–2091
Del Grosso S, Parton W, Stohlgren T, Zheng D, Bachelet D, Prince S, Hibbard K, Olson R (2008) Global potential net primary production predicted from vegetation class, precipitation, and temperature. Ecology 89:2117–2126
Denef K, Six J, Bossuyt H, Frey SD, Elliott ET, Merckx R, Paustian K (2001) Influence of dry-wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol Biochem 33:1599–1611
Dermody O, Weltzin JF, Engel EC, Allen P, Norby RJ (2007) How do elevated [CO2], warming, and reduced precipitation interact to affect soil moisture and LAI in an old field ecosystem. Plant Soil 301:255–266
Didden WAM (1993) Ecology of terrestrial Enchytraeidae. Pedobiologia 37:2–29
Drake BG, Gonzàlez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Phys 48:609–639
Drigo B, Kowalchuk GA, Van Veen JA (2008) Climate change goes underground: effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biol Fert Soils 44:667–679
Elliott ET, Anderson RV, Coleman DC, Cole CV (1980) Habitable pore space and microbial trophic interactions. Oikos 35:327–335
Field CB, Jackson RB, Mooney HA (1995) Stomatal responses to increased CO2: implications from the plant to the global scale. Plant Cell Environ 18:1214–1225
Fierer N, Schimel JP (2002) Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34:777–787
Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009) Global patterns in belowground communities. Ecol Lett 12:1238–1249
Freckman DW, Whitford WG, Steinberger Y (1987) Effect of irrigation on nematode population dynamics and activity in desert soils. Biol Fert Soils 3:3–10
Gerten D, Luo Y, Maire Le et al (2008) Modelled effects of precipitation on ecosystem carbon and water dynamics in different climatic zones. Glob Change Biol 14:2365–2379
Goverde M, Erhardt A, Niklaus PA (2002) In situ development of a satyrid butterfly on calcareous grassland exposed to elevated carbon dioxide. Ecology 83:1399–1411
Griffiths BS (1986) Mineralization of nitrogen and phosphorus by mixed cultures of the ciliate protozoan Colpoda steinii, the nematode Rhabditis sp. and the bacterium Pseudomonas fluorescens. Soil Biol Biochem 18:637–641
Gulledge J, Schimel JP (1998) Moisture control over atmospheric CH4 consumption and CO2 production in diverse Alaskan soils. Soil Biol Biochem 30:1127–1132
Gunderson CA, O’Hara KH, Campion CM, Walker AV, Edwards NT (2010) Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Glob Change Biol 16:2272–2286
Haimi J, Laamanen J, Penttinen R, Räty M, Koponen S, Kellomäki S, Niemelä P (2005) Impacts of elevated CO2 and temperature on the soil fauna of boreal forests. Appl Soil Ecol 30:104–112
Hairston N, Smith F, Slobodkin L (1960) Community structure, population control, and competition. Am Nat 94:421–425
Harrington R, Woiwod I, Sparks T (1999) Climate change and trophic interactions. Trends Ecol Evol 14:146–150
Harte J, Rawa A, Price V (1996) Effects of manipulated soil microclimate on mesofaunal biomass and diversity. Soil Biol Biochem 28:313–322
Hassink J, Bouwman LA, Zwart KB, Brussaard L (1993) Relationships between habitable pore space, soil niota and mineralization rates in grassland soils. Soil Biol Biochem 25:47–55
Hattori T (1988) Soil aggregates in microhabitats of microorganisms. Rep Inst Agric Res Tohoku Univ 37:23–36
Hedlund K, Öhrn MS (2000) Tritrophic interactions in a soil community enhance decomposition rates. Oikos 88:585–591
Heemsbergen DA, Berg MP, Loreau M, van Hal JR, Faber JH, Verhoef HA (2004) Biodiversity effects on soil processes explained by interspecific functional dissimilarity. Science 306:1019–1020
Hendrix PF, Parmelee RW, Crossley DA, Coleman DC, Odum EP, Groffman PM (1986) Detritus food webs in conventional and no-tillage agroecosystems. Bioscience 36:374–380
Hodkinson ID, Healey V, Coulson S (1994) Moisture relationships of the high arctic collembolan Onychiurus arcticus. Physiol Entomol 19:109–114
Hoeksema JD, Lussenhop J, Teeri JA (2000) Soil nematodes indicate food web responses to elevated atmospheric CO2. Pedobiologia 44:725–735
Hughes L (2000) Biological consequences of global warming: is the signal already. Trends Ecol Evol 15:56–61
Hungate BA, Jaeger CH III, Gamara G, Chapin FS III, Field CB (2000) Soil microbiota in two grasslands: responses to elevated atmospheric CO2. Oecologia 124:589–598
Hungate BA, Stiling PD, Dijkstra P, Johnson DW, Ketterer ME, Hymus GI, Hinkle CR, Drake BG (2004) CO2 elicits long-term decline in nitrogen fixation. Science 304:1291
Hungate BA, van Groenigen KJ, Six J, Jastrow JD, Luo Y, de Graaff MA, van Kessel C, Osenberg CW (2009) Assessing the effect of elevated carbon dioxide on soil carbon: a comparison of four meta-analyses. Glob Change Biol 15:2020–2034
Hunt HW, Coleman DC, Ingham ER et al (1987) The detrital food web in a shortgrass prairie. Biol Fert Soils 3:57–68
Ingham RE, Trofymow JA, Ingham ER, Coleman DC (1985) Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol Monogr 55:119–140
Ingham ER, Trofymow JA, Ames RN, Hunt HW, Morley CR, Moore JC, Coleman DC (1986) Trophic interactions and nitrogen cycling in a semi-arid grassland soil. I. Seasonal dynamics of the natural populations, their interactions and effects on nitrogen cycling. J Appl Ecol 23:597–614
Jastrow JD, Miller RM, Matamala R, Norby RJ, Boutton TW, Rice CW, Owensby CE (2005) Elevated atmospheric carbon dioxide increases soil carbon. Glob Change Biol 11:2057–2064
Jones FGW, Larbey DW, Parrott DM (1969) The influence of soil structure and moisture on nematodes, especially Xiphinema, Longidorus, Trichodorus and Heterodera spp. Soil Biol Biochem 1:153–165
Kardol P, Reynolds WN, Norby RJ, Classen AT (2011) Climate change effects on soil microarthropod abundance and community structure. Appl Soil Ecol 47:37–44
Keith DM, Johnson EA, Valeo C (2010) Moisture cycles of the forest floor organic layer (F and H layers) during drying. Water Resour Res 46:W07529. doi:10.1029/2009WR007984
Kirschbaum MUF (2004) Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss? Glob Change Biol 10:1870–1877
Klironomos JN, Rillig MC, Allen MF (1996) Below-ground microbial and microfaunal responses to Artemisia tridentate grown under elevated atmospheric CO2. Funct Ecol 10:527–534
Klironomos JN, Rillig MC, Allen MF, Zak DR, Kubiske M, Pregitzer KS (1997) Soil fungal-arthropod responses to Populus temuloides grown under enriched atmospheric CO2 under field conditions. Glob Change Biol 3:473–478
Klironomos JN, Allen MF, Rillig MC, Piotrowski J, Makvandi-Nejad S, Wolfe BE, Powell JR (2005) Abrupt rise in atmospheric CO2 overestimates community response in a model plant–soil system. Nature 433:621–624
Kuikman PJ, Van Veen JA (1989) The impact of protozoa on the availability of bacterial nitrogen to plants. Biol Fert Soils 8:13–18
Kuikman PJ, Van Vuuren MMI, Van Veen JA (1989) Effect of soil moisture regime on predation by protozoa of bacterial biomass and the release of bacterial nitrogen. Agric Ecosyst Environ 27:271–279
Laakso J, Setälä H, Palojärvi A (2000) Influence of decomposer food web structure and nitrogen availability on plant growth. Plant Soil 225:153–165
Lavelle P, Bignell D, Lepage M, Wolters V, Roger P, Ineson P, Heal OW, Dhillion S (1997) Soil function in a changing world: the role of invertebrate ecosystem engineers. Eur J Soil Biol 33:159–193
Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–1771
Lensing JR, Wise DH (2006) Predicted climate change alters the indirect effect of predators on an ecosystem process. Proc Natl Acad Sci USA 103:15502–15505
Lensing JR, Wise DH (2007) Impact of changes in rainfall amounts predicted by climate-change models on decomposition in a deciduous forest. Appl Soil Ecol 35:523–534
Lindberg N, Persson T (2004) Effects of long-term nutrient fertilization and irrigation on the microarthropod community in a boreal Norway spruce stand. For Ecol Manag 188:125–135
Lindberg N, Engtsson JB, Persson T (2002) Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J Appl Ecol 39:924–936
Luo Y (2001) Transient ecosystem responses to free-air CO2 enrichment (FACE): experimental evidence and methods of analysis. New Phytol 152:3–8
Luo Y, Wan S, Hui D, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413:622–625
Luo Y, Su B, Currie WS et al (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739
Meyer WM, Ostertag R, Cowie RH (2010) Macro-invertebrates accelerate litter decomposition and nutrient release in a Hawaiian rainforest. Soil Biol Biochem 43:206–211
Morgan JA, Pataki DE, Körner C, Clark H, Del Grosso SJ, Grünzweig JM, Knapp AK, Mosier AR, Newton PCD, Niklaus PA, Nippert JB, Nowak RS, Parton WJ, Polley HW, Shaw MR (2004) Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140:11–25
Neher DA, Weicht TR, Moorhead DL, Sinsabaugh RL (2004) Elevated CO2 alters functional attributes of nematode communities in forest soils. Funct Ecol 18:584–591
Nijs I, Impens I (1996) Effects of elevated CO2 concentration and climate-warming on photosynthesis during winter in Lolium perenne. J Exp Bot 47:915–924
Niklaus PA, Alphei J, Ebersberger D, Kampichler C, Kandeler E, Tscherko D (2003) Six years of in situ CO2 enrichment evoke changes in soil structure and soil biota of nutrient-poor grassland. Glob Change Biol 9:585–600
Niu S, Li Z, Xia J, Han Y, Wu M, Wan S (2008) Climatic warming changes plant photosynthesis and its temperature dependence in a temperate steppe of northern China. Environ Exp Bot 63:91–101
Norby RJ, Luo Y (2004) Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytol 162:281–293. doi:10.1111/j.1469-8137.2004.01047.x
Odhiambo HO, Ong CK, Deans JD, Wilson J, Khan AAH, Sprent JI (2001) Roots, soil water and crop yield: tree crop interactions in a semi-arid agroforestry system in Kenya. Plant Soil 235:221–233
Oren R, Ellsworth DE, Johnsen KH et al (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 411:469–472
Osenberg CW, Sarnelle O, Cooper SD (1997) Effect size in ecological experiments: the application of biological models in meta-analysis. Am Nat 150:798–812
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C (2007) Influences of species, latitudes and methodologies on estimates of phonological response to global warming. Glob Change Biol 13:1860–1872
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Peters RH (1993) A critique for ecology. Cambridge University Press, Cambridge
Petersen H, Luxton M (1982) A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 39:288–388
Ponsard S, Arditi R (2000) What can stable isotopes (δ 15N and δ 13C) tell us about the food web of soil macro-invertebrates? Ecology 81:852–864
Prieto P, Peñuelas J, Llusià J, Asensio D, Estiarte M (2009) Effects of long-term experimental night-time warming and drought on photosynthesis, Fv/Fm and stomatal conductance in the dominant species of a Mediterranean shrubland. Acta Physiol Plant 31:729–739
Rosenberg MS, Adams DC, Gurevitch J (2000) MetaWin Version 2.1: Statistical software for meta-analysis. Sinauer, Boston
Ruess L, Michelsen A, Schmidt IK, Jonasson S (1999) Simulated climate change affecting microorganisms, nematode density and biodiversity in subarctic soils. Plant Soil 212:63–73
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Rutherford PM, Juma NG (1992) Influence of texture on habitable pore space and bacterial-protozoan populations in soil. Biol Fert Soils 12:221–227
Rygiewicz PT, Monleon VJ, Ingham ER, Martin KJ, Johnson MG (2010) Soil life in reconstructed ecosystems: initial soil food web responses after rebuilding a forest soil profile for a climate change experiment. Appl Soil Ecol 45:26–38
Savin MC, Görres JH, Neher DA, Amador JA (2001) Biogeophysical factors influencing soil respiration and mineral nitrogen content in an old field soil. Soil Biol Biochem 33:429–438
Scheu S (2002) The soil food web: structure and perspectives. Eur J Soil Biol 38:11–20
Scheu S, Falca M (2000) The soil food web of two beech forests (Fagus sylvatica) of contrasting humus type: stable isotope analysis of a macro- and a mesofauna-dominated community. Oecologia 123:285–296
Shaw MR, Zavaleta ES, Chiariello NR, Cleland EE, Mooney HA, Field CB (2002) Grassland responses to global environmental changes suppressed by elevated CO2. Science 298:1987–1990
Shi L, Guttenberger M, Kottke I, Hampp R (2002) The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of fungi. Mycorrhiza 12:303–311
Sinclair BJ (2002) Effect of increased temperatures simulating climate change on terrestrial invertebrates on Ross Island, Antarctica. Pedobiologia 46:150–160
Six J, Carpentier A, van Kessel C, Merckx, Harris D, Horwath WR, Lüscher A (2001) Impact of elevated CO2 on soil organic matter dynamics as related to changes in aggregate turnover and residue quality. Plant Soil 234:27–36
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569
Sjursen H, Michelsen A, Holmstrup M (2005) Effects of freeze-thaw cycles on microarthropods and nutrient availability in a sub-Arctic soil. Appl Soil Ecol 28:79–93
Sonnemann I, Wolters V (2005) The microfood web of grassland soils responds to a moderate increase in atmospheric CO2. Glob Change Biol 11:1148–1155
Sowerby A, Emmett B, Beier C, Tietema A, Peñuelas J, Estiarte M, Van Meeteren MJM, Hughes S, Freeman C (2005) Microbial community changes in heathland soil communities along a geographical gradient: interaction with climate change manipulations. Soil Biol Biochem 37:1805–1813
Staddon PL, Fitter AH (1998) Does elevated atmospheric carbon dioxide affect arbuscular mycorrhizas? Trends Ecol Evol 13:455–458
Stiling P, Cornelissen T (2007) How does elevated carbon dioxide (CO2) affect plant–herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Glob Change Biol 13:1823–1842
Strickland MS, Rousk J (2010) Considering fungal:bacterial dominance in soils–methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395
Strong DT, De Wever H, Merckx R, Recous S (2004) Spatial location of carbon decomposition in the soil pore system. Eur J Soil Sci 55:739–750
Todd TC, Blair JM, Milliken GA (1999) Effects of altered soil–water availability on a tallgrass prairie nematode community. Appl Soil Ecol 13:45–55
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Tsiafouli MA, Kallimanis AS, Katana E, Stamou GP, Sgardelis SP (2005) Responses of soil microarthropods to experimental short-term manipulations of soil moisture. Appl Soil Ecol 29:17–26
Van Gestel M, Merckx R, Vlassak K (1996) Spatial distribution of microbial biomass in microaggregates of a silty-loam soil and the relation with the resistance of microorganisms to soil drying. Soil Biol Biochem 28:503–510
Van Veen JA, Paul EA (1979) Conversion of biovolume measurements of soil organisms, grown under various moisture tensions, to biomass and their nutrient content. Appl Environ Microbiol 37:686–692
Van Vliet PCJ, Beare MH, Coleman DC (1995) Population dynamics and functional roles of Enchytraeidae (Oligochaeta) in hardwood forest and agricultural ecosystems. Plant Soil 170:199–207
Vargas R, Hattori T (1986) Protozoan predation of bacterial cells in soil aggregates. FEMS Microbiol Ecol 38:233–242
Verhoef HA, Brussaard L (1990) Decomposition and nitrogen mineralization in natural and agroecosystems: the contribution of soil animals. Biogeochemistry 11:175–211
Wall DH, Bradford MA, St. John MG et al (2008) Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob Change Biol 14:2661–2677
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Princeton University Press, Princeton
Weltzin JF, Loik ME, Schwinning S et al (2003) Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 53:941–952
West AW, Sparling GP, Speir TW, Wood JM (1988) Dynamics of microbial-C, N-flush and ATP, and enzyme activities of gradually dried soils from a chronosequence. Aust J Soil Res 26:519–530
West AW, Sparling GP, Feltham CW, Reynolds J (1992) Microbial activity and survival in soil dried at different rates. Aust J Soil Res 30:209–222
Woods LE, Cole CV, Elliott ET, Anderson RV, Coleman DC (1982) Nitrogen transformations as affected by bacterial–microfaunal interactions. Soil Biol Biochem 14:93–98
Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198:97–107
Wright DA, Killham K, Glover LA, Prosser JI (1995) Role of pore size location in determining bacterial activity during predation by Protozoa in soil. Appl Environ Microbiol 61:3537–3543
Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA (2010) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob Change Biol. doi:10.1111/j.1365-2486.2010.02302.x
Yeates GW, Bongers T, de Goede RG, Freckman DW, Georgieva SS (1993) Feeding habits in soil nematode families and genera—an outline for soil ecologists. J Nematol 25:315–331
Yeates GW, Tate KR, Newton PCD (1997) Response of the fauna of a grassland soil to doubling of atmospheric carbon dioxide concentration. Biol Fert Soils 25:307–315
Yeates GW, Newton PCD, Ross DJ (2003) Significant changes in soil microfauna in grazed pasture under elevated carbon dioxide. Biol Fert Soils 38:319–326
Zak DR, Pregitzer KS, King JS, Holmes WE (2000) Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol 147:201–222
Zogg GP, Zak DR, Ringelberg DB, MacDonald NW, Pregitzer KS, White DC (1997) Compositional and functional shifts in microbial communities due to soil warming. Soil Sci Soc Am J 61:475–481
Acknowledgments
Thanks to helpful comments from participants, including J. Gurevitch and M. Rosenberg, during the organized oral session on "Synthesizing ecological studies in a changing world using meta-analysis" at the Summer 2007 ESA Meeting in San Jose, CA, USA. Thanks to B. Duval and T. Wojtowicz for their help in editing and adding clarity. Thanks to S. Hart, M. Watwood, and the invaluable comments from four anonymous reviewers from previous drafts of this manuscript. This work was supported by the US National Science Foundation (DEB-0092642).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jason Kaye.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blankinship, J.C., Niklaus, P.A. & Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 165, 553–565 (2011). https://doi.org/10.1007/s00442-011-1909-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-1909-0