Abstract
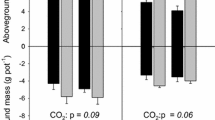
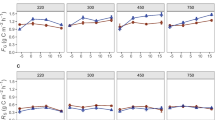
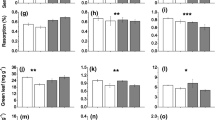
Carbon allocation and N acquisition by plants following defoliation may be linked through plant–microbe interactions in the rhizosphere. Plant C allocation patterns and rhizosphere interactions can also be affected by rising atmospheric CO2 concentrations, which in turn could influence plant and microbial responses to defoliation. We studied two widespread perennial grasses native to rangelands of western North America to test whether (1) defoliation-induced enhancement of rhizodeposition would stimulate rhizosphere N availability and plant N uptake, and (2) defoliation-induced enhancement of rhizodeposition, and associated effects on soil N availability, would increase under elevated CO2. Both species were grown at ambient (400 μL L−1) and elevated (780 μL L−1) atmospheric [CO2] under water-limiting conditions. Plant, soil and microbial responses were measured 1 and 8 days after a defoliation treatment. Contrary to our hypotheses, we found that defoliation and elevated CO2 both reduced carbon inputs to the rhizosphere of Bouteloua gracilis (C4) and Pascopyrum smithii (C3). However, both species also increased N allocation to shoots of defoliated versus non-defoliated plants 8 days after treatment. This response was greatest for P. smithii, and was associated with negative defoliation effects on root biomass and N content and reduced allocation of post-defoliation assimilate to roots. In contrast, B. gracilis increased allocation of post-defoliation assimilate to roots, and did not exhibit defoliation-induced reductions in root biomass or N content. Our findings highlight key differences between these species in how post-defoliation C allocation to roots versus shoots is linked to shoot N yield, but indicate that defoliation-induced enhancement of shoot N concentration and N yield is not mediated by increased C allocation to the rhizosphere.









Similar content being viewed by others
References
Allard V, Robin C, Newton PCD, Lieffering M, Soussana JF (2006) Short and long-term effects of elevated CO2 on Lolium perenne rhizodeposition and its consequences on soil organic matter turnover and plant N yield. Soil Biol Biochem 38:1178–1187
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bardgett RD, Wardle DA, Yeates GW (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 30:1867–1878
Bement RE (1969) A stocking-rate guide for beef production on blue-grama range. J Range Manage 22:83–86
Biondini ME, Klein DA, Redente EF (1988) Carbon and nitrogen losses through root exudation by Agropyron cristatum, A. smithii and Bouteloua gracilis. Soil Biol Biochem 20:477–482
Briske DD, Richards JH (1995) Plant responses to defoliation: a physiological, morphological and demographic evaluation. In: Bedunah D, Sosebee R (eds) Wildland plants: physiological ecology and developmental morphology. Society for Range Management, Denver, pp 635–710
Briske DD, Boutton TW, Wang Z (1996) Contribution of flexible allocation priorities to herbivory tolerance in C4 perennial grasses: an evaluation with 13C lablelling. Oecologia 105:151–159
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Bruulsema TW, Duxbury JM (1996) Simultaneous measurement of soil microbial nitrogen, carbon, and carbon isotope ratio. Soil Sci Soc Am J 60:1787–1791
Burke IC, Lauenroth WK, Riggle R, Brannen P, Madigan B, Beard S (1999) Spatial variability of soil properties in the shortgrass steppe: the relative importance of topography, grazing, microclimate, and plant species in controlling spatial patterns. Ecosystems 2:422–438
Caldwell MM, Richards JH, Johnson DA, Nowak RS, Dzurec RS (1981) Coping with herbivory: photosynthetic capacity and resource allocation in two semiarid Agropyron bunchgrasses. Oecologia 50:14–24
Chapin F, McNaughton S (1989) Lack of compensatory growth under phosphorus deficiency in grazing-adapted grasses from the Serengeti Plains. Oecologia 79:551–557
Cheng W (1999) Rhizosphere feedbacks in elevated CO2. Tree Physiol 19:313–320
Clark NM, Apple ME, Nowak RS (2010) The effects of elevated CO2 on root respiration rates of two Mojave Desert shrubs. Glob Chang Biol 16:1566–1575
Detling J, Painter E (1983) Defoliation responses of western wheatgrass populations with diverse histories of prairie dog grazing. Oecologia 57:65–71
Detling JK, Dyer MI, Winn DT (1979) Net photosynthesis, root respiration, and regrowth of Bouteloua gracilis following simulated grazing. Oecologia 41:127–134
Diaz S, Grime JP, Harris J, McPherson E (1993) Evidence of a feedback mechanism limiting plant response to elevated carbon dioxide. Nature 364:616–617
Dijkstra FA, Pendall E, Mosier AR, King JY, Milchunas DG, Morgan JA (2008) Long-term enhancement of N availability and plant growth under elevated CO2 in a semi-arid grassland. Funct Ecol 22:975–982
Dijkstra FA, Blumenthal DM, Morgan JA, Pendall E, Carrillo Y, Follett R (2010) Contrasting effects of elevated CO2 and warming on nitrogen cycling in a semiarid grassland. New Phytol 187:426–437
Dilkes NB, Jones DL, Farrar J (2004) Temporal dynamics of carbon partitioning and rhizodeposition in wheat. Plant Physiol 134:706–715
Eneboe EJ, Sowell BF, Heitschmidt RK, Karl MG, Haferkamp MR (2002) Drought and grazing IV: blue grama and western wheatgrass. J Range Manage 55:73–79
Fu S, Cheng W (2004) Defoliation affects rhizosphere respiration and rhizosphere priming effect on decomposition of soil organic matter under a sunflower species: Helianthus annuus. Plant Soil 263:345–352
Goverde M, Erhardt A (2003) Effects of elevated CO2 on development and larval food-plant preference in the butterfly Coenonympha pamphilus (Lepidoptera, Satyridae). Glob Chang Biol 9:74–83
Guitian R, Bardgett RD (2000) Plant and soil microbial responses to defoliation in temperate semi-natural grassland. Plant Soil 220:271–277
Guo J, Trotter CM, Newton PCD (2006) Initial observations of increased requirements for light-energy dissipation in ryegrass (Lolium perenne) when source/sink ratios become high at a naturally grazed free air CO2 enrichment (FACE) site. Funct Plant Biol 33:1045–1053
Hamilton EW, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–2402
Hamilton EW, Giovannini MS, Moses SJ, Coleman JS, McNaughton SJ (1998) Biomass and mineral element responses of a Serengeti short grass species to nitrogen supply and defoliation: compensation requires a critical [N]. Oecologia 114:407–418
Hamilton EW, Frank DA, Hinchey P, Murray T (2008) Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Biol Biochem 40:2865–2873
Henry F, Vestberg M, Christensen S (2008) Evidence for a transient increase of rhizodeposition within one and a half day after a severe defoliation of Plantago arenaria grown in soil. Soil Biol Biochem 40:1264–1267
Hu S, Chapin FS, Firestone MK, Field CB, Chiariello NR (2001) Nitrogen limitation of microbial decomposition in a grassland under elevated CO2. Nature 409:188–191
Hungate BA, Lund CP, Pearson HL, Chapin FS (1997) Elevated CO2 and nutrient addition alter soil N cycling and N trace gas fluxes with early season wet-up in a California annual grassland. Biogeochemistry 37:89–109
Ilmarinen K, Mikola J, Vestberg M (2008) Do interactions with soil organisms mediate grass responses to defoliation? Soil Biol Biochem 40:894–905
Jacob J, Greinter C, Drake BG (1995) Acclimation of photosynthesis in relation to Rubisco and non-structural carbohydrate contents and in situ carboxylase activity in Scirpus olneyi grown at elevated CO2 in the field plant. Cell Environ 18:875–884
Johansson EM, Fransson PMA, Finlay RD, van Hees PAW (2009) Quantitative analysis of soluble exudates produced by ectomycorrhizal roots as a response to ambient and elevated CO2. Soil Biol Biochem 41:1111–1116
Karn JF, Ries RE (2002) Free-choice grazing of native range and cool-season grasses. J Range Manage 55:469–473
King JY, Mosier AR, Morgan JA, LeCain DR, Milchunas DG, Parton WJ (2004) Plant nitrogen dynamics in shortgrass steppe under elevated atmospheric carbon dioxide. Ecosystems 7:147–160
Lauenroth WK, Sala OE, Milchunas DG, Lathrop RW (1987) Root dynamics of Bouteloua gracilis during short-term recovery from drought. Funct Ecol 1:117–124
Lauenroth WK, Burke IC, Gutmann MP (1999) The structure and function of ecosystems in the central North American grassland region. Gt Plains Res 9:223–260
LeCain DR, Morgan JA, Mosier AR, Nelson JA (2003) Soil and plant water relations determine photosynthetic responses of C3 and C4 grasses in a semi-arid ecosystem under elevated CO2. Ann Bot 92:41–52
Luo Y et al (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739
Mawdsley JL, Bardgett RD (1997) Continuous defoliation of perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) and associated changes in the composition and activity of the microbial population of an upland grassland soil. Biol Fertil Soils 24:52–58
McCulley RL, Burke IC, Lauenroth WK (2009) Conservation of nitrogen increases with precipitation across a major grassland gradient in the Central Great Plains of North America. Ecosyst Ecol 159:571–581
Menke JW, Trlica MJ (1981) Carbohydrate reserve, phenology, and growth cycles of nine Colorado range species. J Range Manage 34:269–277
Mikola J, Kytoviita MM (2002) Defoliation and the availability of currently assimilated carbon in the Phleum pratense rhizosphere. Soil Biol Biochem 34:1869–1874
Mikola J, Yeates GW, Barker GM, Wardle DA, Bonner KI (2001) Effects of defoliation intensity on soil food-web properties in an experimental grassland community. Oikos 92:333–343
Milchunas DG, Lauenroth WK (1992) Carbon dynamics and estimates of primary production by harvest, 14C dilution, and 14C turnover. Ecology 73:593–607
Milchunas DG, Mosier AR, Morgan JA, LeCain DR, King JY, Nelson JA (2005a) Elevated CO2 and defoliation effects on a shortgrass steppe: forage quality versus quantity for ruminants. Agric Ecosyst Environ 111:166–184
Milchunas DG, Mosier AR, Morgan JA, LeCain DR, King JY, Nelson JA (2005b) Root production and tissue quality in a shortgrass steppe exposed to elevated CO2: using a new ingrowth method. Plant Soil 268:111–122
Milchunas D, Lauenroth W, Burke I, Detling JK (2008) Effects of grazing on vegetation. In: Lauenroth W, Burke IC (eds) Ecology of the shortgrass steppe: a long-term perspective. Oxford University Press, New York, pp 389–446
Morgan JA, LeCain DR, Read JJ, Hunt HW, Knight WG (1998) Photosynthetic pathway and ontogeny affect water relations and the impact of CO2 on Bouteloua gracilis (C4) and Pascopyrum smithii (C3). Oecologia 114:483–493
Morgan JA, Skinner RH, Hanson JD (2001) Nitrogen and CO2 affect regrowth and biomass partitioning differently in forages of three functional groups. Crop Sci 41:78–86
Morgan JA et al (2004a) Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140:11–25
Morgan JA, Mosier AR, Milchunas DG, LeCain DR, Nelson JA, Parton WJ (2004b) CO2 enhances productivity, alters species composition, and reduces digestibility of shortgrass steppe vegetation. Ecol Appl 14:208–219
Niklaus P, Glockler E, Siegwolf R, Korner C (2001) Carbon allocation in calcareous grassland under elevated CO2: a combined 13C pulse-labelling/soil physical fractionation study. Funct Ecol 15:43–50
Paterson E, Thornton B, Sim A, Pratt S (2003) Effects of defoliation and atmospheric CO2 depletion on nitrate acquisition, and exudation of organic compounds by roots of Festuca rubra. Plant Soil 250:293–305
Paterson E, Thornton B, Midwood AJ, Sim A (2005) Defoliation alters the relative contributions of recent and non-recent assimilate to root exudation from Festuca rubra. Plant Cell Environ 28:1525–1533
Polley H, Detling J (1988) Herbivory tolerance of Agropyron smithii populations with different grazing histories. Oecologia 77:261–267
Read JJ, Morgan JA (1996) Growth and partitioning in Pascopyrum smithii (C3) and Bouteloua gracilis (C4) as influenced by carbon dioxide and temperature. Ann Bot 77:487–496
Sala OE, Lauenroth WK, Parton WJ (1992) Long-term soil water dynamics in the shortgrass steppe. Ecology 73:1175–1181
Skinner RH, Morgan JA, Hanson JD (1999) Carbon and nitrogen reserve remobilization following defoliation: nitrogen and elevated CO2 effects. Crop Sci 39:1749–1756
Stitt M (1991) Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ 14:741–762
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wilsey BJ, Coleman JS, McNaughton SJ (1997) Effects of elevated CO2 and defoliation on grasses: a comparative ecosystem approach. Ecol Appl 7:844–853
Ziska LH, Sicher RC, Kremer DF (1995) Reversibility of photosynthetic acclimation of swiss chard and sugarbeet grown at elevated concentrations of CO2. Physiol Plant 95:355–364
Acknowledgments
We thank Dan LeCain for assistance with leaf water potential measurements, experimental set-up and greenhouse maintenance, and Caitlin Brooks, Ally Eden, Erik Hardy, Pat McCusker and David Smith for assistance with plant harvests and laboratory analyses. We thank Daniel Milchunas, Shenglei Fu, Christian Korner, and three anonymous reviewers for thoughtful reviews that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christian Körner.
Rights and permissions
About this article
Cite this article
Augustine, D.J., Dijkstra, F.A., William Hamilton III, E. et al. Rhizosphere interactions, carbon allocation, and nitrogen acquisition of two perennial North American grasses in response to defoliation and elevated atmospheric CO2 . Oecologia 165, 755–770 (2011). https://doi.org/10.1007/s00442-010-1845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1845-4