Abstract
Although interference competition is a conspicuous component of many animal communities, it is still uncertain whether the competitive ability of a species determines its relative abundance and patterns of association with other species. We used replicated arena tests to quantify behavioral dominance of eight common species of co-occurring ground-foraging ants in the Siskiyou Mountains of southern Oregon. We found that behavior recorded in laboratory assays was an accurate representation of a colony’s ability to monopolize resources in the field. We used interaction frequencies from the behavioral tests to estimate transition probabilities in a simple Markov chain model to predict patterns of relative abundance in a metacommunity that is dominated by behavioral interactions. We also tested whether behavioral interactions between each pair of species could be used to predict patterns of species co-occurrence. We found that the Markov model did not accurately predict patterns of observed relative abundance on either the local or the regional scale. However, we did detect a significant negative correlation at the local scale in which behaviorally dominant species occupied relatively few baits. Pairwise behavioral data also did not predict species co-occurrence in any site. Although interference competition is a conspicuous process in ant communities, our results suggest that it may not contribute much to patterns of relative abundance and species co-occurrence in the system studied here. However, the negative correlation between behavioral dominance and bait occupancy at the local scale suggests that competition–colonization trade-offs may be important in resource acquisition and persistence of behaviorally subordinate species.
Similar content being viewed by others
Introduction
A fundamental question in community ecology is how the competitive ability of a species determines its relative abundance in a community and its pattern of co-occurrence with other species (Cody and Diamond 1975; Chase and Leibold 2003). Competitive dominants may achieve high relative abundance because of their ability to sequester and accumulate energy and resources. Many plant communities are organized as transitive competitive hierarchies, in which superior competitors achieve the greatest abundance in the field (reviewed in Keddy 1990). Experimental studies of vertebrate (Heske et al. 1994; Griffis and Jaeger 1998; Trewby et al. 2008) and invertebrate (Race 1982; Kohler 1992; Andersen and Patel 1994) communities provide evidence for competitive release following the exclusion of a dominant, abundant species.
In the presence of a competitive dominant, how do weak competitors persist in a community? Communities may be structured by competition–colonization trade-offs in which stronger competitors may be dispersal limited (Tilman 1994; Levine and Rees 2002). With spatial variation in disturbance regimes, competition–colonization dynamics may create a “successional mosaic” (Chesson and Huntly 1997) of species occupying patches with different disturbance histories (Roxburgh et al. 2004) or becoming abundant at different times in a successional sequence (Wilson 1990). Alternatively, poorer competitors may be better suited to different environmental conditions and may be abundant in different locations across a heterogenous landscape (Cody and Diamond 1975), in different successional stages (Pacala and Rees 1998), or in fluctuating environments (Chesson 2000). Local abundance may also be influenced by processes occurring at the regional scale, such that the immigration of individuals from productive “source” populations rescue populations from local extinction in “sink” populations (Pulliam 1988; Leibold et al. 2004). As a consequence, even though behavioral interactions and interference competition may be conspicuous, there may be no relationship between competitive ability and relative abundance (Duralia and Reader 1993), or even an inverse relationship (Rabinowitz et al. 1984).
Competitive interactions may also contribute to patterns of species co-occurrence. Species may segregate along niche axes (Silvertown 2004) to partition limited resources (Chase and Leibold 2003) and co-occur less often than chance (Gotelli and Graves 1996). Alternatively, if resources are distributed patchily, intraspecific aggregation by superior competitors may facilitate persistence of weaker competitors without distinct spatial segregation of the community (Ives 1988; Inouye 1999). Disturbance and strong habitat filters reduce the species pool (Keddy 1992) which may only allow species with particular traits to occur (Díaz et al. 1999). In assemblages structured by metacommunity dynamics, competition may generate negative or positive covariation in the occurrence of species pairs, depending on the properties of landscape heterogeneity and migration distances (Hanski 2008).
Ant communities are an ideal system for evaluating the role of competition in determining relative abundance and species co-occurrence because there is substantial interference competition expressed through agonistic behavioral interactions (Fellers 1987; Savolainen and Vepsäläinen 1988; Cerdá et al. 1997; LeBrun 2005), and there are several good examples of common or widespread species that are competitively dominant species (Savolainen and Vepsäläinen 1988; Holway 1999; Palmer et al. 2000; Parr et al. 2005). The ant mosaic theory (Leston 1973) predicts that behaviorally dominant species exclude one another and form spatial mosaics, but subordinate species can coexist with dominants. Support for ant mosaic patterns has been found in simple tropical (Majer 1993; Blüthgen and Stork 2007) and boreal (Savolainen and Vepsäläinen 1988) communities.
However, many studies of behavioral dominance are incomplete because some species simply never co-occur together locally, so that not all pairwise behavioral interactions can be observed in the field. As a result, behavioral observations in the field are unable to quantify fully the specific process (behavioral dominance) proposed to drive the given pattern (relative abundance, co-occurrence). Moreover, qualitative patterns of competitive hierarchies do not make quantitative predictions of relative abundance (but see Adler et al. 2007), and full behavioral datasets are rarely used to make mechanistic predictions of statistical patterns of co-occurrence (Cole 1983). In this study, we quantified the behavioral interactions between all possible pairs of species in controlled arena experiments. We use these ethograms to construct a simple Markov replacement model that predicts the relative abundance of species based entirely on their pairwise interactions. We then asked how well this model predicts the observed relative abundance of species occurring at resource patches (bait stations) in the field. We also asked whether the behavioral interactions between each pair of species could be used to predict patterns of species co-occurrence.
Materials and methods
Study area
We sampled ant communities in the Siskiyou Mountains near the Oregon-California border, USA during June–August of 2003 and 2004. This area is part of the Klamath-Siskiyou ecoregion which has a Mediterranean climate, with cool winters (mean January minimum temperature = 0°C) and warm dry summers (mean July maximum temperature = 31.7°C; mean annual precipitation = 154 cm, with only 4 cm falling between June and August). During the summer months, there is a large diurnal variation in temperature (soil surface temperature range of 10–75°C), but relatively little monthly variation (mean monthly temperatures of June–August between 21.1 and 26.6°C). Forests are mostly open stands of Pinus jeffreyi with other sclerophyllous trees reduced to a shrub layer (Whittaker 1960).
Community sampling
Regional-scale sampling
During the summer of 2003, we sampled ant communities in 16 forested sites. Environmental characteristics varied among sites (see Table 1 in Ratchford et al. 2005 for detailed site descriptions and locations), although sample plots within a site were selected for their relative homogeneity in microhabitat and forest cover (Wittman et al. 2010).
We sampled the ant community by establishing in each site an 8 × 8 m sampling grid of 25 bait stations arranged in a 5 × 5 grid with 2-m spacing. Each bait station consisted of two laminated 7.6 × 12.7 cm index cards, one baited with ~5.5 g of tuna and the other with a cotton ball soaked in honey water. Each bait station was censused a total of nine times (three observations in each of three time periods). We began observing bait stations at 08:30, 13:00, and 18:30 hours, and the three observations within each time period were separated by 30 min. During each sampling period, we observed each bait station for approximately 20 s and recorded the number and identity of each species present. Any time heterospecific individuals made physical contact on a bait, we also recorded each individual’s behavioral response (behavioral categorizations defined in “Quantifying Behavior” below). Individual workers were collected at the end of the observation period if they could not be readily identified in the field. Phil Ward at the University of California, Davis, confirmed the species identifications. Voucher specimens were deposited at the University of Tennessee in Knoxville. Nomenclature follows Bolton (2003).
Species occurrences at baits may be dominated by behaviorally aggressive or mass-recruiting species, so that baits can potentially under-sample trophic specialists, solitary foragers, and behaviorally subordinate species. We used several baits in an area and made multiple observations throughout the day to minimize these potential omissions (Bestelmeyer et al. 2000). We supplemented the bait-station data by visually searching the plots during each sampling period, but only one ant species that did not occur at baits, Lasius flavus, was found by visual searching. Thus, we are confident that our sampling strategy captured a full range of foraging activity and adequately sampled the community at the local scale. We were unable to supplement bait data with pitfall or Winkler samples due to the extremely rocky substrate and lack of litter, respectively.
In 2003, we observed a total of 34,942 ants from 24 species and 11 genera from the 16 forested sites (Ratchford et al. 2005). Local richness within a site was usually only 8 species (range = 5–12). Aphaenogaster occidentalis, Camponotus vicinus, Tapinoma sessile, and Temnothorax nevadensis occurred at the majority of sites and collectively constituted between 29 and 86% of the species occurrences observed at bait stations within a site. Less regionally widespread species that locally were numerically dominant included Crematogaster coarctata, Formica subelongata, and F. moki. Data summed over the 16 sites constitute the regional abundance of species. Data from two individual sites, Southside and Whiskey Creek, were also used in the local co-occurrence analyses.
Local-scale sampling
In order to more fully quantify abundance and behavioral interactions, we selected an additional site (=Southeast), approximately 200 m from the Southside site, at which we sampled local abundance more intensively, and quantified behavior among all possible species pairs. In 2004, we used 15 tuna bait stations, separated by 5–20 m, located randomly throughout the Southeast site. Baits were observed seven times over the 12-h period and sampled over 5 weeks. We observed a total of eight species (Aphaenogaster occidentalis, Camponotus vicinus, Crematogaster coarctata, Formica moki, Liometopum luctuosum, Solenopsis molesta, Tapinoma sessile, Temnothorax nevadensis), and the numerically dominant species were F. moki, T. sessile, and T. nevadensis. All species found at the Southeast site were used to test the model predictions of local relative abundance and co-occurrence patterns.
Quantifying behavior
Although most studies use encounters at baits to determine species behavioral dominance, not all the possible species combinations are usually observed in the field. Therefore, as in Cole’s (1983) study of mangrove ants, we experimentally forced all pairwise combinations of the eight species observed at Southeast to interact in standardized behavioral arena contests (8 species = 28 unique species pairs). It was not practical to conduct experiments with all regional species (24 species = 276 unique species pairs); thus, regional analyses only included the top six regionally abundant species within distinct genera (Aphaenogaster occidentalis, Camponotus vicinus, Crematogaster coarctata, Formica subelongata, Tapinoma sessile, Temnothorax nevadensi; see Fig. 4 in Ratchford et al. 2005 for regional rank abundance).
We conducted the pairwise behavior experiment by introducing one worker of each species pair into opposite ends of a piece of 8 cm × 64 mm clear Tygon tubing and sealing the ends of the tube. We ran the behavioral experiment under the average ambient temperature recorded in the field throughout the day (~29°C). We began collecting data when the two individuals first made contact, and we recorded all behavioral interactions for a total of 2 min. We recorded each individual’s behavior at every contact, and all dominant, neutral, and subordinate behaviors were recorded. “Dominant” behavior was defined as any type of attack, which included biting, chasing, lunging, or chemical spray. “Neutral” behavior was defined as no visible change in behavior after contact. “Subordinate” behavior was defined as any type of retreat, which most often was running away but also included spasms or death. Ten trials were conducted for each species pair, and new individuals were used for each trial. To avoid chemical contamination, we used a fresh piece of Tygon tubing for each trial, and washed and dried our hands before every trial. Ant workers were collected with an aspirator from tuna fish baits (which were not part of the abundance censuses), and were used in behavioral trials within 3 h of field collection. Whenever possible, we used individuals collected from different colonies of the same species in consecutive trials. Workers of most species were monomorphic, but for C. vicinus, we used major workers in behavioral trials.
With sufficient replication, one-on-one behavioral contests can produce similar results to bioassays using higher numbers of interacting ants (Roulston et al. 2003; but see Holway 1999; Tanner 2008). To test whether the behavior of individual ants encountering heterospecifics in our arena experiments was similar to behavior observed in natural field settings (i.e. at baits often with multiple individuals), we calculated a dominance index for each species from both behavioral arena data and observations at baits in the field. We then tested whether these two independent measures of dominance were correlated among species. Because behavioral dominance is defined as successful fighting ability that displaces other species (Cerdá et al. 1997; Parr and Gibb 2010), we used Feller’s (1987) dominance index (DI) to quantify dominant behavior. The DI is calculated for each species as the proportion of interactions in which a species elicited subordinate behavior in another individual. For both the behavioral experiment and observations at baits, the dominance index was defined as the proportion of contacts, summed over all other species, in which it elicited retreating behavior in the other individual. We compared field and experimental DI values with a randomization test for a regression slope (Edgington 1995), implemented in the EcoSim software package (Gotelli and Entsminger 2006). EcoSim’s Standard Regression Module compares the observed slope to the distribution of slopes generated from repeatedly randomizing the order of the x and y observations.
Finally, we investigated whether our behavioral dominance indices measured the ability of a colony to successfully defend resource patches in the field. We calculated “social dominance” (Morse 1974), which is the ability of a colony to access resources through successful fighting abilities. Fighting and behavioral aggression are the mechanisms of interference competition in this system, which does not have any territorial species (sensu Savolainen and Vepsäläinen 1988). We defined social dominance as the proportion of instances after an interspecific encounter in which a species controlled the bait, evident by sustained forager numbers through time and active removal of bait (Bestelmeyer 2000; Yanoviak and Kaspari 2000) for at least three consecutive sample periods (=60 min). A significant correlation between the behavioral dominance measured in the laboratory and social dominance measured in the field suggests the pairwise behavioral assays are representative of a colony’s ability to monopolize resources in nature.
Using behavior to predict relative abundance
Measures of relative abundance
We used two different measures of relative abundance: bait visitation and forager abundance. Bait visitation was defined as the presence of a species on a bait. If a species had at least one worker on a bait during any sampling period, it was counted as one bait visit (maximum value = number of baits per site). Forager abundance was defined as the average number of workers per visited. This measurement does not take into account the total number of baits visited; rather, it describes the ability of a species to successfully recruit to a visited bait.
At the local scale, we averaged abundance values over the 5-week sampling period at the Southeast site. Additionally, at Southeast, during each sampling period, we followed foragers at baits back to their nests and discovered (1) a single bait was never visited by multiple nests of the same species and (2) a single nest never recruited to more than one bait. Repeated observations of the permanent baits stations indicated nest locations were static throughout the 5-week period. Thus, at the local scale, our bait visitation values are also good correlates of nest counts.
At the regional scale, we averaged abundance values of the six most common species over the 16 regional sites. This grand average reflects a species’ ability to recruit to baits across several site types and environmental conditions. At the 16 regional sites, we did not identify nest entrances. However, because foraging distances measured at the Southeast site were generally 2–3 m, and our bait stations were separated by 5 m, bait visitation was a rough approximation for nest density.
Markov chain model
Markov chain models were first introduced to community ecology studies to model succession processes (Waggoner and Stephens 1970) and remain a useful yet under-utilized tool in community ecology (Hill et al. 2004). Markov chain models treat the landscape as a large set of patches which may take on different ecological states (e.g., different species, different size classes, unoccupied space, etc.). How a community changes through time is defined by the probability of one ecological state at time t transitioning to another at time t + 1. These transition probabilities are often derived through repeated sampling of fixed points or patches in which the community state changes with time. Thus, these field-measured transition probabilities do not represent any specific mechanistic process; they are the sum of all direct and indirect process among species (Wootton 2001). Here, we take a different approach: we estimated transition probabilities based on competitive interactions directly from laboratory behavioral assays, and used those probabilities to construct a Markov model. We then used the Markov model to predict the relative abundance of a community based on a specific, isolated process: behavioral dominance. Finally, we tested those model predictions with independent abundance measures from field censuses.
The local model for the Southeast site included eight different states (=species), representing the eight species that were present, and thus its dimensions are 8 × 8. The regional model (=6 species) consisted of a 6 × 6 matrix, representing the six species that were common throughout the region. The entries in both matrices are the transition probabilities that one species will persist or be displaced by a different species in a patch during a single time step. We used the dominance behavior observed in the laboratory experiment to create the transition probabilities. Two types of transition probabilities were calculated. The diagonal elements represent the likelihood that a species replaces itself in the next time step and were estimated as the proportion of times a species did not retreat during its encounters with all other species in the laboratory experiment. The off-diagonal elements represent the probability that a species will turnover in a patch after an encounter with another species. We used the proportion of retreats of one species from another as the off-diagonal values. For example, in Table 2a (below), there is a 49.9% chance that Aphaenogaster occidentalis will replace itself (persist) in a patch, and a 10.4% chance that it will be expelled by Crematogaster coarctata.
Matrices are column-stochastic (each column sums to 1.0; Caswell 2006), because entries are the probability of transition from one state of occupancy of one species to another. The input vector, representing initial species abundance, was set at 1,000 individuals for each species. The model was run for 1,000 time steps until the stable state distribution was achieved, which corresponds to the first eigenvector of the transition matrix (Caswell 2006). Because the “states” in this model represent the different species, the distribution of individuals among states can be interpreted as the relative abundance of each species at an equilibrium that is determined by the probabilistic outcomes of the all the pairwise species interactions. The Markov model predicts the relative abundance of each species in the assemblage, and we tested whether this prediction was significantly correlated with the observed abundance of the same species in the local and regional field surveys.
We also modified the Markov model to test its sensitivity to the estimate of persistence probability. In this alternative model, we isolated expulsion behavior as the sole driver of relative abundance by substituting a zero for each diagonal value in the transition probability matrix, and re-scaled so matrices were still column stochastic. In this model, species turnover was determined exclusively by the off-diagonal values, which represent the probability that one species can expel another. Results were qualitatively similar to the original Markov model with non-zero diagonal elements and therefore are not reported.
Using behavior to predict species co-occurrence
Null model
We tested whether agonistic behavior predicted species co-occurrence in three local ant assemblages: Southside and Whiskey Creek, 2 of the 16 sites sampled in 2003, and Southeast, the repeatedly sampled site in 2004. These three sites are occupied by a similar suite of species, occur within 0.5 km of each other, yet have slightly different site characteristics (see Table 1 in Ratchford et al. 2005).
To evaluate species co-existence patterns, we calculated the C-score (Stone and Roberts 1990) which is the number of ‘checkerboard units’ of each species pair. For each species pair, the checkerboard index is (r a –S)(r b –S) where S is the total number of ‘sites’ (=baits) shared by the species pair, and r a and r b are the row totals for species a and b, respectively. Species that always occur together will have a C-score of zero. The greater the segregation in species occurrences, the larger will be the C-score. Results are reported in terms of the standard effect size (SES) which scales the results in terms of standard deviations (Gurevitch et al. 1992). Large positive SES values (>1.96) indicate significant species segregation. Large negative SES values (<−1.96) indicate species aggregation (Gotelli 2000).
Assemblages were randomized using the fixed-equiprobable model in EcoSim, version 7 (Gotelli and Entsminger 2006). In this null model, the columns represented the baits (n = 15 for the Southeast site, n = 25 for the Southside and Whiskey Creek sites) and were equally suitable for species. However, the row totals, which represent the total number of baits occupied by a species, were fixed and set to be equivalent to the observed row totals. Thus, species occurred in the same frequency in the randomized as in the observed assemblage, but observed species richness per bait was not constrained. Presence–absence matrices were generated for a single evening sampling period, because this is the time of day when non-random species co-occurrence values are most likely to occur (Wittman et al. 2010). The model was run separately for each unique species pair observed during this time (n = 15 species pairs for site Southeast, n = 10 species pairs for sites Southside and Whiskey Creek).
To address the potential effect of temperature on patterns of behavioral dominance and species co-occurrence, we also conducted co-occurrence analyses for each species pair at each site during warm (morning) and hot (afternoon) times of day. Our analyses using these data yielded similar results to the cool, evening period, and therefore are not reported.
We used pairwise DI values generated from the behavior experiment (described above) as the predictor variable and the SES value for each corresponding null model test of the occurrence data as the response variable in a randomization test of the regression slope (Gotelli and Entsminger 2006). Because each point represents a unique species pair, each DI value is the sum of the proportion of contacts in which one species caused the other species to retreat. Thus, the DI value ranges from 0 (both species never retreated in the presence of the other) to 2 (both species repelled each other at every contact). If species interactions determine co-occurrence patterns, species pairs with a strong tendency to repel each other are predicted to be segregated in occurrence (large positive SES values), whereas species pairs that tolerate each other are predicted to be aggregated (large negative SES values) or random in occurrence (small SES values).
Results
Field and experimental dominance indices
The behavioral arena experiment utilized all species found at the Southeast site and repeatedly tested all species combinations, resulting in a complete behavioral dataset based on hundreds of interactions. This is in contrast to observations based in the field, in which only 9 of 28 possible species pairs were observed. Unique species pairs repeatedly were observed an average of seven times at sites in the field (range = 1–18) whereas each species pair was observed an average of 104 times in the behavioral experiment (range = 26–279). Because field observations also incorporate differences in recruitment abilities, more individuals were present during a field encounter (average = 21 workers) than during an experimental encounter (always two workers). Although multiple individuals were often present at a bait, workers physically interacted with only one individual at a time. See Table 1 for the summary of differences between field observations and the behavioral experiment. Table 1 reports details for behavioral observations at the single local site (=Southeast; 525 total bait observations); details of field observations (fraction of all species pairs observed, number of observations per species pair, number of individuals present during interactions) are similar when summed over all regional sites (=16 sites; 3,600 total bait observations; Wittman, unpublished data).
Although the behavioral experiment used one-on-one interactions in unbaited tubes, experimental DIs were similar in magnitude to those calculated from species occurrences on baits in the field, which usually included several individuals of both species. Field and laboratory estimates of DI were significantly correlated at the local scale (mean of simulated slopes = −0.002, observed slope = 0.187, P = 0.024) and positive but marginally non-significant for behaviors summed across multiple sites (mean of simulated slopes = −0.039, observed slope = 1.80, P = 0.092; Fig. 1). Dominance indices based on field observations were always larger than those based on experimental arena data (Fig. 1). Collectively, these results suggest that behavioral interactions measured in the laboratory experiment are comparable to those observed in field encounters at resource patches.
The relationship between experimental and field based dominance values for local (solid line) and regional (dashed line) species sets. Each point represents a species dominance index as determined by repeated interactions with all local species (closed markers) or the most common regional species (open markers). The local behavioral experiment relationship is significant (P = 0.024), whereas the regional relationship is marginally non-significant (P = 0.092). AO Aphaenogaster occidentalis, CC Crematogaster coarctata, CV Camponotus vicinus, FM Formica moki, FS F. subelongata, LL Liometopum luctuosum, SM Solenopsis molesta, TS Tapinoma sessile, TN Temnothorax nevadensis
Measures of individual pairwise dominance indices in the laboratory predicted colony-level social dominance as observed in the field. A total of 27 interspecific encounters were observed at baits at the Southeast site. Of these, 8 could not be scored for an outcome because they occurred in the terminal census, and in 2 cases, neither species dominated the bait. For the remaining 17 cases, behavioral dominance (measured in the arena experiments) correlated with the social dominance (ability of colonies to defend and use resources in the field; Fig. 2). Individual behavior measured both in the laboratory (mean of simulated slopes = 0.008, observed slope = 0.841, P = 0.008) and observed the field (mean of simulated slopes = 0.011, observed slope = 2.190, P = 0.019) predicted colony-level behavioral dominance of baits (Fig. 2).
The relationship between the pairwise dominance index, the ability of one individual worker to expel another, and social dominance, the ability of a species to expel other species and dominate a bait in the field. Each point represents a species. Both laboratory (open markers) and field (closed makers) dominance indices predict the capacity of a colony to defend baits successfully (P = 0.019 and 0.011, respectively). See Fig. 1 for species abbreviations
Behavioral prediction of relative abundance
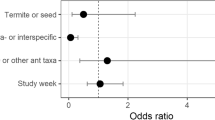
We used the data from the behavior experiments to generate two transition probabilities matrices (Table 2), one local scale and one regional scale, to predict relative abundance. On neither spatial scale did the Markov model accurately predict the number of workers of each species at occupied baits (Fig. 3b, d). At the regional scale, the Markov model also did not accurately predict the fraction of occupied baits (Fig. 3a). In fact, the only statistically significant result was opposite of the Markov model predictions: at the local scale, behaviorally dominant species were significantly less likely to occupy baits (mean of simulated slopes = 0.017, observed slope = −0.762, P = 0.008; Fig. 3c).
The relationship between predicted relative abundance (based on Markov behavioral dominance model) and observed relative abundance, measured as (a) proportion of baits occupied and (b) worker numbers on the regional scale and (c) proportion of baits occupied and (d) worker numbers on the local scale. The dashed line indicates a perfect model fit between the model predictions and the field data, and the solid line indicates the least-squares slope for the fitted relationship. P values show results of a one-tailed randomization test for slopes differing from 0.0
Behavioral prediction of species co-occurrence
Several species pairs exhibited non-random co-occurrence patterns across the three sites (Table 3). Temnothorax nevadensis was the only species which showed significant segregation, and it was usually segregated from mass-recruiting species (e.g., C. coarctata, L. luctuosum, T. sessile). The only significant aggregation occurred between T. nevadensis and F. moki. Although we detected non-random co-occurrence patterns at all sites, behavioral dominance indices did not predict co-occurrence patterns observed at the Southeast site (mean of simulated slopes = −0.002, observed slope = −0.334, P = 0.339; Fig. 4a), the Southside site (mean of simulated slopes = 0.100, observed slope = 3.218, P = 0.168; Fig. 4b) or the Whiskey Creek site (mean of simulated slopes = −0.002, observed slope = −0.334, P = 0.339; Fig. 4c).
The relationship between experimental dominance indices and field co-occurrence values. Each point represents a unique species pair. Co-occurrence values are expressed as standard effect sizes, which scale results in terms of standard deviations. Values above 1.96 (dashed line) indicate significant segregation while values below −1.96 (dashed line) indicate significant aggregation. Behavioral interactions did not predict co-occurrence (slope test P > 0.05) at either the (a) Southeast site, (b) Southside site, or (c) Whiskey Creek site
Discussion
Although interference competition is a conspicuous element of ant community ecology, our models using complete pairwise dominance data among all members of the local community did not predict patterns of relative abundance (Fig. 3) or species co-occurrence (Fig. 4). Laboratory measurements of behavior accurately represented behavior in the field: behaviorally dominant species identified in laboratory contests also successfully supplanted other species at baits (Fig. 1), and in doing also monopolized food resources (Fig. 2). However, the ability of a species to successfully dominate baits was not linked to forager numbers (Fig. 3b, d) or the occupation of many baits within a site (Fig. 3a, c). Indeed, our local-scale results indicate behaviorally dominant species occupy relatively few baits (Fig. 3c). Thus, unlike communities found in the arid tropics (Andersen and Patel 1994), boreal taiga (Savolainen and Vepsäläinen 1989), mangrove islands (Cole 1983), or rainforest canopy (Morrison 1996), ant communities in the Siskiyou mountains do not appear to host ecologically dominant species (sensu Davidson 1998) that are both behaviorally and numerically dominant.
The ecological impact of behaviorally dominant ants may be tempered by their decreased ability to locate food resources (the dominance/discovery trade-off; Fellers 1987; Davidson 1998), physiological constraints that limit foraging (behavioral dominance/thermal tolerance trade-off; Cerdá et al. 1997; Bestelmeyer 2000), greater vulnerability to predators or parasites (Feener 2000; LeBrun and Feener 2007), or reduced colonization ability (Stanton et al. 2002). Ants in the Siskiyou Mountains do not show a trade-off in exploitation and interference competition, and are not subject to parasitoid attacks while foraging (Wittman 2007). Although there is no clear behavioral dominance–thermal tolerance trade-off (Wittman 2007), thermal tolerance does underlie foraging activity, with stronger competitive interactions occurring during cooler portions of the day (Wittman et al. 2010). However, even when averaging foraging abundances over the entire diurnal range in temperature (worker numbers) or using abundance metrics unrelated to worker numbers (nest counts), behavioral dominance could not predict relative abundance (Fig. 3).
Adler et al.’s (2007) model is one of the few that incorporated explicit dominance diversity tradeoffs, but it also did not successfully predict relative abundance of ant communities in the Chiricahua Mountains. Its inability to successfully predict relative abundance may have been due to population size effects on dominance (e.g., Palmer 2004), non-equilibrium effects of environmental fluctuations and migration, or nest site limitation (Adler et al. 2007). We found dominance indices were similar using varying numbers of workers (Fig. 1), indicating that behavioral dominance in this community may not be sensitive to differences in colony size. However, our local-scale results (Fig. 3c) demonstrated that behaviorally dominant species occupied relatively few baits (=nest sites, see “Measures of relative abundance”). This pattern suggests trade-offs in competition–colonization (Stanton et al. 2002) may decouple behavioral dominance from abundance observed in the field (e.g., Palmer et al. 2000) and provide a mechanism for the persistence of subordinate species. Further study is needed to measure directly colonization rates to examine if behaviorally dominant species are indeed poorer colonizers.
Our arena behavioral assays would be suspect if they did not reflect colony-level behavior as it is expressed in the field. For example, one-on-one behavioral experiments did not predict dominance in the field in the invasive argentine ant (Linepithema humile) because its individual and colony level behavioral dominance differ (Holway 1999). Additionally, behavioral dominance quantified in laboratory arenas may not be representative when behavior is dependent upon previous experience with competitors, neutrality of location, or value of resource (Tanner and Adler 2009) or is temperature-dependent (Cerdá et al. 1997). However, the results of our behavioral arena experiments were consistent with field observations recorded under varying thermal conditions with varying numbers of workers (Fig. 1). Thus, the laboratory contests allowed us to accurately quantify behavior among all species pairs, and to provide data on 19 out of 28 species pairs unattainable simply through field observations (Table 1).
That experimental dominance indices were consistently smaller than those based on observations at baits (Figs. 1 and 2) was not surprising given the large number of replicates and total number of interactions observed in the arena experiment. However, because the Markov models are column stochastic, they are not affected by the absolute size of the DI indices, only by their relative values among species pairs. Finally, the dominance indices used to parameratize the Markov model were representative of colony-level abilities to defend and access resources in the field (Fig. 2), which incorporate differences in recruitment strategies, body size differences, and changing environmental conditions. Collectively, our approach provided a complete, replicated dataset of behavioral interactions, which represents the outcome of colony-level defense of resource patches in nature.
We found behavioral dominance did not predict patterns of co-occurrence in any of the ant communities studied (Fig. 4). That is, strongly antagonistic species are not more likely than other species to segregate across baits. Evidence of agonistic behavior underlying co-occurrence patterns is well documented in ant communities, including tropical plantations (Majer 1993; Blüthgen and Stork 2007), boreal forests (Savolainen and Vepsäläinen 1988), and small islands (Cole 1983). Although we found variation in the co-occurrence patterns of species pairs across sites (Table 3), the outcome of behavioral interactions between species pairs did not predict co-occurrence patterns (Fig. 4). Because ant mosaics often involve the tending of honeydew-producing hemipterans that fuel territorial behavior (Davidson 1997; Blüthgen et al. 2000; Davidson et al. 2004), ant mosaics may not be found in assemblages of opportunistic scavengers (this study) because they would be guarding a space with a variable rate of resource appearance (Styrsky and Eubanks 2007).
The only species that regularly displayed non-random associations with other species was T. nevadensis, which often segregated with mass-recruiting species and aggregated with one species F. moki (Table 3). A similar pattern was noted by Savolainen and Vepsäläinen (1989), who found Leptothorax sp. (many Leptothorax have recently been reclassified in the genus Temnothorax; Bolton 2003) positively associating with Formica spp. Fellers (1987) also found that Leptothorax coexisted more often on baits with several other larger species. As in these other studies, T. nevadensis may aggregate with F. moki. due to its ‘insinuator’ behavior (Wilson 1971): when this small ant encounters another species, it freezes and brings its antennae close to its head, sometimes even lying on its side. Other larger species often ignore it while they are feeding on the bait, and after a few minutes, T. nevadensis will try to resume foraging, often gathering small pieces of food that are created by the shredding activity of larger foraging species. This subordinate behavior and size-selective feeding may also explain why T. nevadensis coexisted infrequently with species such as C. coarctata, L. luctuosum, and T. sessile (Table 3), all of which recruit many small workers that stay on baits for several hours.
Although ant communities are generally thought to be strongly structured by interference competition, recent removal experiments have shown weak effects of competitively dominant species on other community members (Gibb and Hochuli 2004; King and Tschinkel 2006). These experimental results and the analyses presented here suggest that behaviorally dominant species may not always have a large impact on the abundance and occurrence of subordinate species. For ant communities in the Siskiyou Mountains, the quantitative predictions of simple Markov models based on standardized behavioral assays usually did not accurately predict relative abundance or co-occurrence patterns. In the one case in which the model did predict species occurrence at the local scale, behavioral dominance was negatively correlated with occurrence, suggesting that behaviorally dominant species are rare in spite of, not because of, their competitive abilities (Rabinowitz et al. 1984).
References
Adler FR, LeBrun EG, Feener DH (2007) Maintaining diversity in an ant community: modeling, extending, and testing the dominance-discovery trade-off. Am Nat 169:323–333
Andersen AN, Patel AD (1994) Meat ants as dominant members of Australian ant communities–an experimental test of their influence on the foraging success and forager abundance of other species. Oecologia 98:15–24
Bestelmeyer BT (2000) The trade-off between thermal tolerance and behavioural dominance in a subtropical South American ant community. J Anim Ecol 69:998–1009
Bestelmeyer BT, Agosti D, Alonso LE, Brandao CRF, Brown WL, Delabie JHC, Silvestre R (2000) Field techniques for the study of ground-dwelling ants: an overview, description and evaluation. Smithsonian Institution Press, Washington D.C.
Blüthgen N, Stork NE (2007) Ant mosaics in a tropical rainforest in Australia and elsewhere: a critical review. Austral Ecol 32:93–104
Blüthgen N, Verhaagh M, Goitía W, Jaffé K, Morawetz W, Barthlott W (2000) How plants shape the ant community in the Amazonian rainforest canopy: the key role of extrafloral nectaries and homopteran honeydew. Oecologia 125:229–240
Bolton B (2003) Synopsis and classification of Formicidae. Mem Am Entomol Inst 71:1–370
Caswell H (2006) Matrix population models: construction, analysis, and interpretation, 2nd edn. Sinauer, Sunderland
Cerdá X, Retana J, Cros S (1997) Thermal disruption of transitive hierarchies in Mediterranean ant communities. J Anim Ecol 66:363–374
Chase JM, Leibold LA (2003) Ecological niches. University of Chicago Press, Chicago
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Chesson P, Huntly N (1997) The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am Nat 150:519–553
Cody ML, Diamond JM (1975) Ecology and evolution of communities. Harvard University Press, Cambridge
Cole BJ (1983) Assembly of mangrove ant communities–patterns of geographical distribution. J Anim Ecol 52:339–347
Davidson DW (1997) The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol J Linn Soc 61:153–181
Davidson DW (1998) Resource discovery versus resource domination in ants: a functional mechanism for breaking the trade-off. Ecol Entomol 23:484–490
Davidson DW, Cook S, Snelling R (2004) Liquid feeding performances of ants (Formicidae): ecological and evolutionary implications. Oecologia 139:255–266
Díaz S, Cabido M, Casanoves F (1999) Functional implications of trait-environment linkages in plant communities. In: Weiher E, Keddy PA (eds) Ecological assembly rules: perspectives, advances, retreats. Cambridge University Press, Cambridge, pp 338–362
Duralia TE, Reader RJ (1993) Does abundance reflect competitive ability–a field test with three prairie grasses. Oikos 68:82–90
Edgington ES (1995) Randomization Tests. Marcel Dekker, New York
Feener DH (2000) Is the assembly of ant communities mediated by parasitoids? Oikos 90:79–88
Fellers JH (1987) Interference and exploitation in a guild of woodland ants. Ecology 68:1466–1478
Gibb H, Hochuli D (2004) Removal experiment reveals limited effects of a behaviorally dominant species on ant assemblages. Ecology 85:648–657
Gotelli NJ (2000) Null model analysis of species co-occurrence patterns. Ecology 81:2606–2621
Gotelli NJ, Entsminger GL (2006) EcoSim: null models software for ecology. Version 7.0. Acquired Intelligence Inc. & Kesey-Bear. http://www.garyentsminger.com/ecosim/ecosim.htm, Jericho, VT 05465
Gotelli NJ, Graves GR (1996) Null models in ecology. Smithsonian Institution Press, Washington
Griffis MR, Jaeger RG (1998) Competition leads to an extinction-prone species of salamander: interspecific territoriality in a metapopulation. Ecology 79:2494–2502
Gurevitch J, Morrow L, Wallace A, Walsh J (1992) A meta-analysis of competition field experiments. Am Nat 140:539–572
Hanski I (2008) Spatial patterns of coexistence of competing species in patchy habitat. Theor Ecol 1:29–43
Heske EJ, Brown JH, Mistry S (1994) Long-term experimental study of a Chihuahuan Desert rodent community: 13 years of competition. Ecology 75:438–445
Hill MF, Witman JD, Caswell H (2004) Markov chain analysis of succession in a rocky subtidal community. Am Nat 164:E46–E61
Holway D (1999) Competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology 80:238–251
Inouye BD (1999) Integrating nested spatial scales: implications for the coexistence of competitors on a patchy resource. J Anim Ecol 68:150–162
Ives AR (1988) Aggregation and the coexistence of competitors. Ann Zool Fenn 25:75–88
Keddy PA (1990) Competitive hierarchies and centrifugal organization in plant communities. In: Grace J, Tilman D (eds) Perspectives on plant competition. Academic, San Diego, pp 266–290
Keddy PA (1992) Assembly and response rules: two goals for predictive community ecology. J Veg Sci 3:157–164
King JR, Tschinkel WR (2006) Experimental evidence that the introduced fire ant, Solenopsis invicta, does not competitively suppress co-occurring ants in a disturbed habitat. J Anim Ecol 5:1370–1378
Kohler SL (1992) Competition and the structure of a benthic stream community. Ecol Monogr 62:165–188
LeBrun EG (2005) Who is the top dog in ant communities? Resources, parasitoids, and multiple competitive hierarchies. Oecologia 142:643–652
LeBrun EG, Feener DH (2007) When trade-offs interact: balance of terror enforces dominance discovery trade-off in a local ant assemblage. J Anim Ecol 76:58–64
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Leston D (1973) The ant mosaic–tropical tree crops and the limiting of pests and diseases. Proc Natl Acad Sci USA 19:311–341
Levine JM, Rees M (2002) Coexistence and relative abundance in annual plant assemblages: the roles of competition and colonization. Am Nat 160:452–467
Majer JD (1993) Comparison of the arboreal ant mosaic in Ghana, Brazil, Papua New Guinea and Australia–its structure and influence on arthropod diversity. CAB International, Wallingford
Morrison LW (1996) Community organization in a recently assembled fauna: the case of Polynesian ants. Oecologia 107:243–256
Morse DH (1974) Niche breadth as a function of social dominance. Am Nat 108:818–830
Pacala SW, Rees M (1998) Models suggesting field experiments to test two hypotheses explaining successional diversity. Am Nat 152:729–737
Palmer TM (2004) Wars of attrition: colony size determines competitive outcomes in a guild of African acacia ants. Anim Behav 68:993–1004
Palmer TM, Young TP, Stanton ML, Wenk E (2000) Short-term dynamics of an acacia ant community in Laikipia, Kenya. Oecologia 123:425–435
Parr C, Gibb H (2010) Competition and the role of dominant ants. In: Lach L, Parr C, Abbott K (eds) Ant ecology. Oxford University Press, Oxford, pp 77–96
Parr C, Sinclair B, Andersen A, Gaston K, Chown S (2005) Constraint and competition in assemblages: a cross-continental and modeling approach for ants. Am Nat 165:481–494
Pulliam HR (1988) Sources, sinks, and population regulation. Am Nat 132:652–661
Rabinowitz D, Rapp JK, Dixon PM (1984) Competitive abilities of sparse grass species–means of persistence or cause of abundance. Ecology 65:1144–1154
Race MS (1982) Competitive displacement and predation between introduced and native mud snails. Oecologia 54:337–347
Ratchford JS, Wittman SE, Jules ES, Ellison AM, Gotelli NJ, Sanders NJ (2005) The effects of fire, local environment and time on ant assemblages in fens and forests. Divers Distrib 11:487–497
Roulston TH, Buczkowski G, Silverman J (2003) Nestmate discrimination in ants: effect of bioassay on aggressive behavior. Insect Soc 50:151–159
Roxburgh SH, Shea K, Wilson JB (2004) The intermediate disturbance hypothesis: patch dynamics and mechanisms of species coexistence. Ecology 85:359–371
Savolainen R, Vepsäläinen K (1988) A competition hierarchy among boreal ants–impact on resource partitioning and community structure. Oikos 51:135–155
Savolainen R, Vepsäläinen K (1989) Niche differentiation of ant species within territories of the wood ant Formica polyctena. Oikos 56:3–16
Silvertown J (2004) Plant coexistence and the niche. Trends Ecol Evol 19:605–611
Stanton ML, Palmer TM, Young TP (2002) Competition-colonization trade-offs in a guild of African Acacia-ants. Ecol Monogr 72:347–363
Stone L, Roberts A (1990) The checkerboard score and species distributions. Oecologia 85:74–79
Styrsky JD, Eubanks MD (2007) Ecological consequences of interactions between ants and honeydew-producing insects. Proc R Soc Lond B 274:151–164
Tanner CJ (2008) Aggressive group behaviour in the ant Formica xerophila is coordinated by direct nestmate contact. Anim Behav 76:1335–1341
Tanner CJ, Adler FR (2009) To fight or not to fight: context-dependent interspecific aggression in competing ants. Anim Behav 77:297–305
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Trewby LD, Wilson GJ, Delahay RJ, Walker N, Young R, Davison J, Cheeseman C, Robertson PA, Gorman ML, McDonald RA (2008) Experimental evidence of competitive release in sympatric carnivores. Biol Lett 4:170–172
Waggoner P, Stephens G (1970) Transition probabilities for a forest. Nature 255:1160–1161
Whittaker RH (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr 30:279–338
Wilson E (1971) The insect societies. Belknap, Cambridge
Wilson JB (1990) Mechanisms of species co-existence: twelve explanations for Hutchinson’s “Paradox of the plankton”: evidence from New Zealand plant communities. N Z J Ecol 13:17–42
Wittman SE (2007) Ant community assembly in the Siskiyou-Klamath ecoregion. PhD dissertation, University of Vermont, Burlington
Wittman SE, Sanders NJ, Ellison AM, Jules ES, Ratchford JS, Gotelli NJ (2010) Species interactions and thermal constraints on ant community structure. Oikos 119:551–559
Wootton JT (2001) Prediction in complex communities: analysis of empirically derived Markov models. Ecology 82:580–598
Yanoviak SP, Kaspari M (2000) Community structure and the habitat templet: ants in the tropical forest canopy and litter. Oikos 89:259–266
Acknowledgments
We thank N. Kaczmar, R. Lacy, and P. Wittman for assistance with the pairwise behavioral experiments and T. Hart for assistance with the Markov model analysis. Project design and execution was greatly improved through discussions with N. Sanders. Portions of this research were funded by NSF grant 03-01381 to N.J.G.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bernhard Stadler.
Rights and permissions
About this article
Cite this article
Wittman, S.E., Gotelli, N.J. Predicting community structure of ground-foraging ant assemblages with Markov models of behavioral dominance. Oecologia 166, 207–219 (2011). https://doi.org/10.1007/s00442-010-1813-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1813-z