Abstract
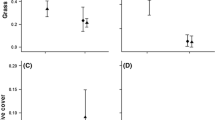
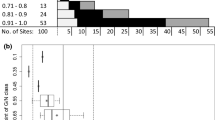
In some plant populations, the availability of seeds strongly regulates recruitment. However, a scarcity of germination microsites, granivory or density-dependent mortality can reduce the number of plants that germinate or survive to flower. The relative strengths of these controls are unknown for most plant populations and for exotic invaders in particular. We conducted a seed addition experiment with a granivore exclusion treatment in a field setting to explore how these factors interact to regulate populations of the widespread invader Centaurea solstitialis (yellow starthistle) at three study sites across the plant’s range in California. We coupled the experimental approach with observational studies within established C. solstitialis populations to estimate seed rain, recruitment and mortality at natural densities. Seed limitation occurred in both experimental and observational plots in all populations. Although vertebrate granivores were active at each site, they had no effect on C. solstitialis recruitment. Density increased mortality, but the effect was variable and weak relative to its effect on fecundity. The seed limitation that was evident at the seedling stage persisted to flowering. Seed-limited populations such as these ought to be highly sensitive to losses to seed predators, and many biological control agents, including those established for C. solstitialis, are seed predators. However, flowering plant density was decoupled from seed production by a strong compensatory response in the surviving plants; seed production was nearly constant in plots across all seed addition levels. Thus, flowering plant density is reduced by the established biocontrol agents, but seed production compensates to replace the population every generation, and no long-term decline is predicted.





Similar content being viewed by others
References
Agrawal AA, Kotanen PM (2003) Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecol Lett 6:712–715
Andersen AN (1989) How important is seed predation to recruitment in stable populations of long-lived perennials? Oecologia 81:310–315
Baker HG (1965) Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species. Academic, London, pp 147–172
Benefield CB, DiTomaso JM, Kyser GB, Tschohl A (2001) Reproductive biology of yellow starthistle (Centaurea solstitialis): maximizing late season control. Weed Sci 49:38–90
Blaney CS, Kotanen PM (2001) Post-dispersal losses to seed predators: an experimental comparison of native and exotic old field plants. Can J Bot 79:284–292
Brown JH, Stevens GC, Kaufman DM (1996) The geographic range: size, shape, boundaries and internal structure. Annu Rev Ecol Syst 27:597–623
Burdon JJ, Chilvers GS (1982) Host density as a factor in plant disease ecology. Annu Rev Phytopathol 20:143–166
Clark JS, Macklin E, Wood L (1998) Stages and spatial scales of recruitment limitation in southern Appalachian forests. Ecol Monogr 68:213–235
Clark CJ, Poulsen JR, Levey DJ, Osenberg CW (2007) Are plant populations seed limited? A critique and meta-analysis of seed addition experiments. Am Nat 170:128–142
Condit R, Hubbell SP, Foster RB (1994) Density dependence in two understory tree species in a neotropical forest. Ecology 75:671–680
Coombs EM, Clark JK, Piper GL, Confrancesco AF (2004) Biological control of invasive plants in the United States, 1st edn. Oregon State University Press, Corvallis
Crawley MJ (1989) Insect herbivores and plant population dynamics. Annu Rev Entomol 34:531–564
Crawley MJ (1992) Seed predators and plant population dynamics. In: Fenner M (ed) Seeds, the ecology of regeneration in plant communities. CAB International, Wallingford, pp 157–192
Crawley MJ (1997) Plant ecology, 2nd edn. Blackwell, London
Crawley MJ, Gillman MP (1989) Population dynamics of cinnabar moth and ragwort in grassland. J Anim Ecol 58:1035–1050
DiTomaso JM, Gerlach JD (2000) Centaurea solstitialis. In: Bossard CC, Randall JM, Hoshovsky MC (eds) Invasive plants of California’s Wildlands. University of California, Berkeley, pp 101–106
DiTomaso JM, Healy EA (2007) Weeds of California and other western states, 1st edn. University of California Press, Oakland
Garren JM, Strauss SY (2009) Population-level compensation by an invasive thistle thwarts biological control from seed predators. Ecol Appl 19:709–721
Gilbert G (2002) Evolutionary ecology of plant diseases in natural ecosystems. Annu Rev Phytopathol 40:13–43
Harper JL (1977) The population biology of plants, 1st edn. Academic, London
Herrera CM, Medrano M, Rey PJ, Sanchez-Lafuente AM, Garcia MB, Guitian J, Manzaneda AJ (2002) Interaction of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism- and antagonism-related traits. Proc Natl Acad Sci USA 99:16823–16828
Hoffmann JH, Moran VC (1998) The population dynamics of an introduced tree, Sesbania punicea, in South Africa, in response to long-term damage caused by different combinations of three species of biological control agents. Oecologia 114:343–348
Hulme PE (1994) Rodent post-dispersal seed predation in grassland: magnitude and sources of variation. J Ecol 82:645–652
Hunter MD (1992) Interactions within herbivore communities mediated by the host plant: the keystone herbivore concept. In: Hunter MD, Ohgushi T, Price PW (eds) Effects of resource distribution on animal–plant interactions. Academic, New York, pp 288–325
Joley DB, Maddox DM, Supkoff DM, Mayfield A (1992) Dynamics of yellow starthistle Centaurea solstitialis achenes in field and laboratory. Weed Sci 40:190–194
Jongejans E, Shea K, Skarpaas O, Kelly D, Sheppard AW, Woodburn TL (2008) Dispersal and demography contributions to populations spread of Carduus nutans in its native and invaded ranges. J Ecol 96:687–697
Kelly D, McCallum K (1995) Evaluating the impact of Rhinocyllus conicus on Carduus nutans in New Zealand. In: Delfosse ES, Scott RR (eds) Proceedings of the 8th International Symposium on Biological Control of Weeds. DSIR/CSIRO, Lincoln University, Lincoln, New Zealand, pp 205–211
Louda SM (1982) Limitation of the recruitment of the shrub Haplopappus squarrosus (Asteraceae) by flower feeding and seed feeding insects. J Ecol 70:43–54
Louda SM (1983) Seed predations and seedling mortality in the recruitment of a shrub Haplopappus venetus (Asteraceae) along a climatic gradient. Ecology 64:511–521
Louda SM (1989) Predation in the dynamics of seed regulation. In: Leck MA, Parker VT, Simpson RL (eds) Ecology of soil seedbanks. Academic, London, pp 25–52
Louda SM, Potvin MA (1995) Effect of inflorescence-feeding insects on the demography and lifetime fitness of a native plant. Ecology 76:229–245
Maron JL, Crone E (2006) Herbivory: effects on plant abundance, distribution and growth. Proc R Soc Biol 273:2575–2584
Maron JL, Simms EL (2001) Rodent-limited establishment of bush lupine: field experiments on the cumulative effect of granivory. J Ecol 89:578–588
Noble IR, Weiss PW (1989) Movement and modelling of buried seed of the invasive perennial Chrysanthemoides monilifera in coastal dunes and biological control. Aust J Ecol 14:55–64
Nuñez MA, Simberloff D, Relva MA (2008) Seed predation as a barrier to alien conifer invasions. Biol Invasions 10:1389–1398
Parker IM (2001) Safe site and seed limitation in Cytisus scoparius (Scotch broom): invasibility, disturbance, and the role of cryptogams in a glacial outwash prairie. Biol Invasions 3:323–332
Parker JD, Hay ME (2005) Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol Lett 8:959–967
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: towards a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461
Pitcairn MJ, O’Connell RA, Gendron JM (1998) Yellow starthistle: survey of statewide distribution. In: Woods DM (ed) Biological control program annual summary 1997. California Department of Food and Agriculture Plant Health and Pest Prevention Services, Sacramento, pp 64–66
Pitcairn MJ, Schoenig S, Yacoub R, Gendron J (2006) Yellow starthistle continues its spread in California. Calif Agric 60:83–90
Poulsen JR, Osenberg CW, Clark CJ, Levey DJ, Bolker BM (2007) Plants as reef fish: fitting the functional form of seedling recruitment. Am Nat 170:167–183
Reichard SH, Hamilton CW (1997) Predicting invasions of woody plants introduced into North America. Conserv Biol 11:193–203
Sheppard AW, Paynter PQ, Rees M (2002) Factors affecting invasion and persistence of broom Cytisus scoparius in Australia. J Appl Ecol 39:721–734
Turnbull LA, Crawley MJ, Rees M (2000) Are plant populations seed-limited? A review of seed sowing experiments. Oikos 88:225–238
van der Meijden E (1979) Herbivore exploitation of a fugitive plant species: local survival and extinction of the cinnabar moth (Tyria jacobaea) and ragwort (Senecio jacobaea) in a heterogeneous environment. Oecologia 42:307–324
Acknowledgments
We gratefully acknowledge the support of Ray Carruthers and the USDA’s Exotic and Invasive Weeds Research Unit. SMS acknowledges the generous support of the Jean Langenheim Fellowship in Plant Ecology and Evolution and an NSF Doctoral Dissertation Improvement Grant (DEB-0808337). Carla D’Antonio, Dan Doak, Laurel Fox and two (very thoughtful) anonymous reviewers provided feedback that improved an earlier version of this manuscript, as did Krikor Andonian, Adelia Barber and Kris Hulvey.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bryan Foster.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Swope, S.M., Parker, I.M. Widespread seed limitation affects plant density but not population trajectory in the invasive plant Centaurea solstitialis . Oecologia 164, 117–128 (2010). https://doi.org/10.1007/s00442-010-1641-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1641-1