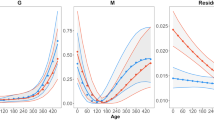
Abstract
A vast literature suggests that maternal factors and egg incubation conditions have substantial effects on offspring phenotypes in oviparous species. However, many studies that evaluate these effects have relied on experimental conditions that are rarely, if ever, encountered under natural conditions. To address this issue, we evaluated relationships among maternal factors, natural nest conditions, egg development in the field, and the resultant offspring phenotypes in a lizard with temperature-dependent sex determination, the jacky dragon (Amphibolurus muricatus, Agamidae). Many, but not all, of the relationships shown in our field-based study corroborate results from laboratory-based experiments. Offspring body size was affected primarily by egg size at oviposition, as well as by water uptake by eggs, rather than by environmental variables measured within the nest. Date of oviposition was related to offspring growth rate and body size prior to the onset of winter; this relationship is likely mediated through an influence on the timing of hatching. Nest temperature generated substantial variation in egg survival; nests that experienced higher temperatures and higher thermal fluctuations suffered relatively high egg mortality. Contrary to results from laboratory incubation, however, nest temperature did not predict offspring sex ratios. Hence, although many results from this field study corroborate those from the laboratory, caution is needed when extrapolating laboratory-incubation results to field conditions.



Similar content being viewed by others
References
Andrews RM, Mathies T, Warner DA (2000) Effect of incubation temperature on morphology, growth, and survival of juvenile Sceloporus undulatus. Herpetol Monogr 14:420–431
Angilletta MJ Jr, Winters RS, Dunham AE (2000) Thermal effects on the energetics of lizard embryos: implications for hatchling phenotypes. Ecology 81:2957–2968
Arnold SJ, Wade MJ (1984) On the measurement of natural and sexual selection; applications. Evolution 38:720–734
Burger J (1976) Temperature relationships in nests of the northern diamondback terrapin, Malaclemys terrapin terrapin. Herpetologica 32:412–418
Buse A, Good JEG, Dury S, Perrins CM (1998) Effects of elevated temperature and carbon dioxide on the nutritional quality of leaves of oak (Quercus robur L.) as food for the winter moth (Operophtera brumata L.). Funct Ecol 12:742–749
Cogger HG (2000) Reptiles and amphibians of Australia. New Holland, Sydney
Crews D, Bull JJ, Wibbels T (1991) Estrogen and sex reversal in turtles: a dose-dependent phenomenon. Gen Comp Endocrinol 81:357–364
Dewitt TJ, Scheiner SM (2004) Phenotypic variation from single genotypes: a primer. In: Dewitt TJ, Scheiner SM (eds) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, Oxford, pp 1–9
Donohue K, Schmitt J (1998) Maternal environmental effects in plants: adaptive plasticity? In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford University Press, Oxford, pp 137–158
Downes SJ, Shine R (1999) Do incubation-induced changes in a lizard’s phenotype influence its vulnerability to predators? Oecologia 120:9–18
Elphick MJ, Shine R (1998) Longterm effects of incubation temperatures on the morphology and locomotor performance of hatchling lizards (Bassiana duperreyi, Scincidae). Biol J Linn Soc 63:429–447
Fischer K, Eenhoorn E, Bot ANM, Brakefield PM, Zwaan BJ (2003) Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc R Soc Lond B 270:2051–2056
Fox CW, Mousseau TA (1998) Maternal effects as adaptations for transgenerational phenotypic plasticity in insects. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford University Press, Oxford, pp 159–177
Frankel OH, Soule ME (1981) Conservation and evolution. Cambridge University Press, Cambridge
Frazier MR, Woods HA, Harrison JF (2001) Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol Biochem Zool 74:641–650
Georges A (1989) Female turtles from hot nests: is it duration of incubation or proportion of development that matters? Oecologia 81:323–328
Georges A (1992) Thermal-characteristics and sex determination in field nests of the pig-nosed turtle, Carettochelys insculpta (Chelonia: Carettochelydidae), from northern Australia. Aust J Zool 40:511–521
Georges A, Doody S, Beggs K, Young J (2004) Thermal models of TSD under laboratory and field conditions. In: Valenzuela N, Lance VA (eds) Temperature-dependent sex determination in vertebrates. Smithsonian Institution, Washington DC, pp 79–89
Georges A, Beggs K, Young JE, Doody SJ (2005) Modelling development of reptile embryos under fluctuating temperature regimes. Physiol Biochem Zool 78:18–30
Harlow PS (1996) A harmless technique for sexing hatchling lizards. Herpetol Rev 27:71–72
Harlow PS, Taylor JE (2000) Reproductive ecology of the jacky dragon (Amphibolurus muricatus): an agamid lizard with temperature-dependent sex determination. Aust Ecol 25:640–652
Janzen FJ (1993) An experimental analysis of natural selection on body size of hatchling turtles. Ecology 74:332–341
Janzen FJ, Ast JC, Paukstis GL (1995) Influence of the hydric environment and clutch on eggs and embryos of two sympatric map turtles. Funct Ecol 9:913–922
Kaplan RH, Phillips PC (2006) Maternal effects and thermally induced plasticity in the frog Bombina orientalis. Evolution 60:142–156
King RB (1993) Determinants of offspring number and size in the brown snake, Storeria dekayi. J Herpetol 27:175–185
Littel RC, Millikin GA, Stoup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute, Cary
Madsen T, Shine R (1996) Determinants of reproductive output in female water pythons (Liasis fuscus: Pythonidae). Herpetologica 52:146–159
Meylan S, Clobert J (2004) Maternal effects on offspring locomotion: influence of density and corticosterone elevation in the lizard Lacerta vivipara. Physiol Biochem Zool 77:450–458
Mousseau TA, Fox CW (1998) Maternal effects as adaptations. Oxford University Press, Oxford
O’Donald P (1970) Measuring change of population fitness by natural selection. Nature 227:307–308
Packard GC, Packard MJ (1988) The physiological ecology of reptilian eggs and embryos. In: Gans C, Huey RB (eds) Biology of the Reptilia, vol 16. Alan R. Liss, New York, pp 523–605
Petraitis PS, Dunham AE, Niewiarowski PH (1996) Inferring multiple causality: the limitations of path analysis. Funct Ecol 10:421–431
Pieau C (1982) Modalities of the action of temperature on sexual differentiation in the field-developing embryos of the European pond turtle Emys orbicularis (Emydidae). J Exp Zool 220:353–360
Radder RS, Shanbhag BA (2004) Factors influencing offspring traits in the oviparous multi-clutched lizard, Calotes versicolor (Agamidae). J Biosci 29:105–110
Radder RS, Warner DA, Cuervo JJ, Shine R (2007) The functional significance of residual yolk in hatchling lizards Amphibolurus muricatus (Agamidae). Funct Ecol 21:302–309
Rhen T, Lang JW (2004) Phenotypic effects of incubation temperature in reptiles. In: Valenzuela N, Lance VA (eds) Temperature-dependent sex determination in vertebrates. Smithsonian Institution, Washington DC, pp 90–98
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
SAS Institute (1997) SAS/STAT user’s guide. Statistical Analysis Systems Institute, Cary
Schwarzkopf L, Brooks RJ (1985) Sex determination in northern painted turtles: effects of incubation at constant and fluctuating temperatures. Can J Zool 63:2543–2547
Shine R, Elphick MJ (2001) The effect of short-term weather fluctuations on temperatures inside lizard nests, and on the phenotypic traits of hatchling lizards. Biol J Linn Soc 72:555–565
Shine R, Elphick MJ, Harlow PS (1997) The influence of natural incubation environments on the phenotypic traits of hatchling lizards. Ecology 78:2559–2568
Shine R, Warner DA, Radder RS (2007) Windows of sexual lability during embryonic development in two lizard species with environmental sex determination. Ecology 88:1781–1788
Sinervo B (1998) Adaptation and maternal effects in the wild: path analysis of natural variation and experimental tests of causation. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford University Press, Oxford, pp 288–306
Sinervo B, De Nardo DF (1996) Costs of reproduction in the wild: path analysis of natural selection and experimental tests of causation. Evolution 50:1299–1313
Sinervo B, Doughty P (1996) Interactive effects of offspring size and timing of reproduction of offspring reproduction: experimental, maternal, and quantitative genetic aspects. Evolution 50:1314–1327
Sinervo B, Huey RB (1990) Allometric engineering: an experimental test of the causes of interpopulational differences in performance. Science 248:1106–1109
Sinervo B, Doughty P, Huey RB, Zamudio K (1992) Allometric engineering: a causal analysis of natural selection on offspring size. Science 258:1927–1930
Spitzer BW (2004) Maternal effects in the soft scale insect Saissetia coffeae (Hemiptera: Coccidae). Evolution 58:2452–2461
Thompson MB (1988) Nest temperatures in the pleurodiran turtle, Emydura macquarii. Copeia 1988:996–1000
Tracy CR (1980) Water relations of parchment-shelled lizard (Sceloporus undulatus) eggs. Copeia 1980:478–482
Warner DA (2007) Amphibolurus muricatus, Predation. Herpetol Rev 38:449
Warner DA, Andrews RM (2002) Laboratory and field experiments identify sources of variation in phenotypes and survival of hatchling lizards. Biol J Linn Soc 76:105–124
Warner DA, Shine R (2005) The adaptive significance of temperature-dependent sex determination: experimental tests with a short-lived lizard. Evolution 59:2209–2221
Warner DA, Shine R (2006) Morphological variation does not influence locomotor performance within a cohort of hatchling lizards (Amphibolurus muricatus, Agamidae). Oikos 114:126–134
Warner DA, Shine R (2007) Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia 154:65–73
Warner DA, Shine R (2008a) Maternal nest-site choice in a lizard with temperature-dependent sex determination. Anim Behav 75:65–73
Warner DA, Shine R (2008b) The adaptive significance of temperature-dependent sex determination in a reptile. Nature 451:566–568
Warner DA, Thomas J, Shine R (2006) A simple and reliable method for attaching radio-transmitters to lizards. Herpetol Conserv Biol 1:129–131
Warner DA, Lovern MB, Shine R (2007) Maternal nutrition affects reproductive output and sex allocation in a lizard with environmental sex determination. Proc R Soc Lond B 274:883–890
Warner DA, Lovern MB, Shine R (2008) Maternal influences of offspring phenotypes and sex ratios in a multi-clutching lizard with environmental sex determination. Biol J Linn Soc 95:256–266
Wilson DS (1998) Nest-site selection: microhabitat variation and its effects on the survival of turtle embryos. Ecology 79:1884–1892
Acknowledgments
We thank T. Bunt, T. Child, and T. Schwartz for assistance radio-tracking lizards in the field. Thanks to F. Janzen and members of his laboratory for comments on an earlier draft of this paper. Thanks to D. Adams for statistical advice. D. A. W. was supported by an International Postgraduate Research Scholarship and an International Postgraduate Award. Funding was provided by the Australian Society of Herpetologists (to D. A. W.) and the Australian Research Council (to R. S.). This project was approved by the University of Sydney Animal Care and Ethics committee (proposal no. L04/5-2005/1/4123) and the New South Wales National Parks and Wildlife Service (permit no. S10658).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Anssi Laurila.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Warner, D.A., Shine, R. Maternal and environmental effects on offspring phenotypes in an oviparous lizard: do field data corroborate laboratory data?. Oecologia 161, 209–220 (2009). https://doi.org/10.1007/s00442-009-1366-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1366-1