Abstract
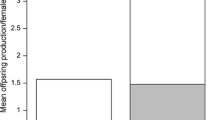
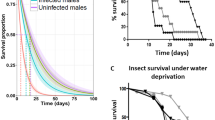
Parasites often produce large numbers of offspring within their hosts. High parasite burdens are thought to be important for parasite transmission, but can also lower host fitness. We studied the protozoan Ophryocystis elektroscirrha, a common parasite of monarch butterflies (Danaus plexippus), to quantify the benefits of high parasite burdens for parasite transmission. This parasite is transmitted vertically when females scatter spores onto eggs and host plant leaves during oviposition; spores can also be transmitted between mating adults. Monarch larvae were experimentally infected and emerging adult females were mated and monitored in individual outdoor field cages. We provided females with fresh host plant material daily and quantified their lifespan and lifetime fecundity. Parasite transmission was measured by counting the numbers of parasite spores transferred to eggs and host plant leaves. We also quantified spores transferred from infected females to their mating partners. Infected monarchs had shorter lifespans and lower lifetime fecundity than uninfected monarchs. Among infected females, those with higher parasite loads transmitted more parasite spores to their eggs and to host plant leaves. There was also a trend for females with greater parasite loads to transmit more spores to their mating partners. These results demonstrate that high parasite loads on infected butterflies confer a strong fitness advantage to the parasite by increasing between-host transmission.



Similar content being viewed by others
References
Altizer SM, Augustine DJ (1997) Interactions between frequency-dependent and vertical transmission in host–parasite systems. Proc Biol Sci 264:807–814
Altizer SM, Oberhauser KS (1999) Effects of the protozoan parasite Ophryocystis elektroscirrha on the fitness of monarch butterflies (Danaus plexippus). J Invertebr Pathol 74:76–88
Altizer SM, Oberhauser KS, Brower LP (2000) Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecol Entomol 25:125–139
Altizer SM, Oberhauser KS, Geurts KA (2004) Transmission of the protozoan parasite, Ophryocystis elektroscirrha, in monarch butterfly populations: implications for prevalence and population-level impacts. In: Oberhauser KS, Solensky M (eds) The monarch butterfly: biology and conservation. Cornell University Press, Ithaca, pp 203–218
Anderson RM, May RM (1982) Coevolution of hosts and parasites. Parasitology 85:411–426
Anderson RM, May RM (1992) Infectious diseases of humans—dynamics and control. Oxford University Press, Oxford
Antia R, Levin BR, May RM (1994) Within-host population dynamics and the evolution and maintenance of microparasite virulence. Am Nat 144:457–472
Bradley CA, Altizer S (2005) Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecol Lett 8:290–300
Bremermann HJ, Pickering J (1983) A game-theoretical model of parasite virulence. J Theor Biol 100:411–426
Brower LP (1995) Understanding and misunderstanding the migration of the monarch butterfly (Nymphalidae) in North America: 1857–1995. J Lepidopt Soc 49:304–385
Bull JJ, Molineux IJ, Rice WR (1991) Selection of benevolence in a host–parasite system. Evolution 45:875–882
Day T (2002) Virulence evolution via host exploitation and toxin production in spore-producing pathogens. Ecol Lett 5:471–476
De Roode JC, Gold LR, Altizer S (2007) Virulence determinants in a natural butterfly–parasite system. Parasitology 134:657–668
De Roode JC, Pedersen AB, Hunter MD, Altizer S (2008a) Host plant species affects virulence in monarch butterfly parasites. J Anim Ecol 77:120–126
De Roode JC, Yates AJ, Altizer S (2008b) Virulence-transmission trade-offs and population divergence in virulence in a naturaly occurring butterfly parasite. Proc Natl Acad Sci USA 105:7489–7494
Diffley P, Scott JO, Mama K, Tsen TN (1987) The rate of proliferation among African trypanosomes is a stable trait that is directly related to virulence. Am J Trop Med Hyg 36:533–540
Ebert D (1999) The evolution and expression of parasite virulence. In: Stearns SC (ed) Evolution in health and disease. Oxford University Press, Oxford, pp 161–172
Ebert D, Zschokke-Rohringer CD, Carius HJ (2000) Dose effects and density-dependent regulation of two microparasites of Daphnia magna. Oecologia 122:200–209
Ewald PW (1983) Host–parasite relations, vectors, and the evolution of disease severity. Annu Rev Ecol Syst 14:465–485
Frank SA (1996) Models of parasite virulence. Q Rev Biol 71:37–78
Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP (2007) Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA 104:17441–17446
Jensen KH, Little TJ, Skorping A, Ebert D (2006) Empirical support for optimal virulence in a castrating parasite. PLoS Biol 4:e197
Leong KL, Yoshimura MA, Kaya HK, Williams H (1997a) Instar susceptibility of the monarch butterfly (Danaus plexippus) to the neogregarine parasite, Ophryocystis elektroscirrha. J Invertebr Pathol 69:79–83
Leong KLH, Yoshimura MA, Kaya HK (1997b) Occurrence of a neogregarine protozoan, Ophryocystis elektroscirrha McLaughlin and Myers, in populations of monarch and queen butterflies. Pan-Pac Entomol 73:49–51
Levin S, Pimentel D (1981) Selection of intermediate rates of increase in parasite–host systems. Am Nat 117:308–315
Lipsitch M, Moxon ER (1997) Virulence and transmissibility of pathogens: what is the relationship? Trends Microbiol 5:31–37
Mackinnon MJ, Read AF (1999) Genetic relationships between parasite virulence and transmission in the rodent malaria Plasmodium chabaudi. Evolution 53:689–703
Mackinnon MJ, Read AF (2004) Virulence in malaria: an evolutionary viewpoint. Philos Trans R Soc Lond B Biol Sci 359:965–986
McLaughlin RE, Myers J (1970) Ophryocystis elektroscirrha sp. n., a neogregarine pathogen of monarch butterfly Danaus plexippus (L.) and the Florida queen butterfly D. gilippus berenice Cramer. J Protozool 17:300–305
Oberhauser KS (1997) Fecundity, lifespan and egg mass in butterflies: effects of male-derived nutrients and female size. Funct Ecol 11:166–175
Oyeyele SO, Zalucki MP (1990) Cardiac glycosides and oviposition by Danaus plexippus on Asclepias fruticosa in south-east Queensland (Australia), with notes on the effect of plant nitrogen content. Ecol Entomol 15:177–185
Prysby MD, Oberhauser K (2004) Temporal and geographic variation in monarch densities: citizen scientists document monarch population patterns. In: Oberhauser K, Solensky M (eds) The monarch butterfly: biology and conservation. Cornell University Press, Ithaca
Stewart AD, Logsdon JM, Kelley SE (2005) An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 59:730–739
Turner CM, Aslam N, Dye C (1995) Replication, differentiation, growth and the virulence of Trypanosoma brucei infections. Parasitology 111:289–300
Urquhart FA, Urquhart NR (1978) Autumnal migration routes of the eastern population of the monarch butterfly (Danaus p. plexippus L.; Danaidae; Lepidoptera) in North America to the overwintering site in the Neovolcanic Plateau of Mexico. Can J Zool 56:1759–1764
Van Baalen M, Sabelis MW (1995) The dynamics of multiple infection and the evolution of virulence. Am Nat 146:881–910
Van Hook T (1993) Non-random mating in monarch butterflies overwintering in Mexico. In: Zalucki MP (ed) Biology and conservation of the monarch butterfly. Natural History Museum of Los Angeles County, Los Angeles, pp 49–60
Vickerman D, Michels A, Burrowes PA (1999) Levels of infection of migrating monarch monarch butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) by the parasite Ophryocystis elektroscirrha (Neogregarinidae: Ophryocystidae), and evidence of a new mode of spore transmission between adults. J Kans Entomol Soc 72:124–128
Zalucki MP (1981) The effects of age and weather on egg laying in Danaus plexippus L. (Lepidoptera: Danaidae). Res Popul Ecol (Kyoto) 23:318–327
Acknowledgments
We thank B. Ledbetter, C. Norman and A. Pedersen for help with the experiments, A. Tull and the UGA Plant Biology greenhouse staff for access to field plots and greenhouse space, and two anonymous referees for helpful comments. J. C. de Roode was supported by a Netherlands Organization for Scientific Research (NWO) TALENT fellowship, a Marie Curie Outgoing International Fellowship and Emory University. S. Altizer was supported by the University of Georgia and NSF DEB-0643831. The experiments comply with current US laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Konrad Fiedler.
Rights and permissions
About this article
Cite this article
de Roode, J.C., Chi, J., Rarick, R.M. et al. Strength in numbers: high parasite burdens increase transmission of a protozoan parasite of monarch butterflies (Danaus plexippus). Oecologia 161, 67–75 (2009). https://doi.org/10.1007/s00442-009-1361-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1361-6