Abstract
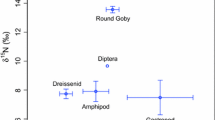
Dreissenid mussels (Dreissena polymorpha and D. bugensis) have re-engineered Great Lakes ecosystems since their introduction in the late 1980s. Dreissenids can have major indirect impacts on profundal habitats by redirecting nutrients and energy away from pelagic production (which supplies profundal production) and depositing nutrients and energy in the nearshore zones that they occupy. However, strong empirical evidence for the effects of this redirection of resources on fish populations is currently lacking. Here, we report significant shifts in isotopic signatures, depth distribution and diets of a coldwater profundal fish population that are all consistent with a greater reliance on nearshore resources after the establishment of dreissenid mussels in South Bay, Lake Huron. Isotopic signatures of scales collected from 5-year-old lake whitefish (Coregonus clupeaformis) demonstrated remarkable stability over the 50-year period prior to the establishment of dreissenids (1947–1997) and a sudden and significant change in isotopic signatures (3‰ enrichment in δ13C and 1‰ depletion in δ15N) after their establishment (2001–2005). These dramatic shifts in isotopic signatures were accompanied by a coincident shift in the mean depth of capture of lake whitefish towards the nearshore. A comparison of previously unpublished pre-invasion diets of lake whitefish from South Bay with contemporary diets collected between 2002 and 2005 also indicate a greater reliance on nearshore prey after the invasion of dreissenid mussels. This study is the first to report changes in the carbon source available to lake whitefish associated with restructured benthic communities after the appearance of dreissenid mussels. Further, this study contributes to a growing body of work that demonstrates the ecological insights that can be gained through isotopic analysis of archived fish bony tissues in ecosystems that have experienced significant levels of disturbance.








Similar content being viewed by others
References
Barbiero RP, Tuchman ML (2004) Long-term dreissenid impacts on water clarity in Lake Erie. J Gt Lakes Res 30:557–565
Casselman JM, Collins JJ, Crossman EJ, Ihssen PE, Spangler GR (1981) Lake whitefish (Coregonus clupeaformis) stocks of the Ontario waters of Lake Huron. Can J Fish Aquat Sci 38:1772–1789
Cochran WG (1977) Sampling techniques. Wiley, New York
Depew DC, Guildford SJ, Smith REH (2006) Nearshore-offshore comparison of chlorophyll a and phytoplankton production in the dreissenid-colonized eastern basin of Lake Erie. Can J Fish Aquat Sci 63:1115–1129
Dermott R (2001) Sudden disappearance of the amphipod Diporeia from Eastern Lake Ontario, 1993–1995. J Gt Lakes Res 27:423–433
Dermott R, Kerec D (1997) Changes to the deepwater benthos of eastern Lake Erie since the invasion of Dreissena: 1979–1993. Can J Fish Aquat Sci 54:922–930
Edsall TA (1999) Preferred temperatures of juvenile lake whitefish. J Gt Lakes Res 25:583–588
Fernandez RJ, Rennie, MD, Sprules, WG (In press) Changes in nearshore zooplankton associated with species invasions and potential effects on larval lake whitefish (Coregonus clupeaformis). Int Rev Hydrobiol
Forseth IN, Innis AF (2004) Kudzu (Pueraria montana): History, physiology, and ecology combine to make a major ecosystem threat. Crit Rev Plant Sci 23:401–413
Foster SE (2007) Co-occurrence and interactions of large invertebrate predators in relation to the Bythotrephes invasion. Ph.D. thesis, University of Toronto, Toronto
France RL (1995) Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol Oceanogr 40:1310–1313
France RL (1998) Density-weighted δ13C analysis of detritivory and algivory in littoral macroinvertebrate communities of boreal headwater lakes. Ann Zool Fenn 35:187–193
Gatz DF, Smith L (1995) The standard error of a weighted mean concentration. 1. Bootstrapping versus other methods. Atmos Environ 29:1185–1193
Gerdeaux D, Perga ME (2006) Changes in whitefish scales δ13C during eutrophication and reoligotrophication of subalpine lakes. Limnol Oceanogr 51:772–780
Hart JL (1931) The food of the whitefish (Coregonus clupeaformis) in Ontario waters, with a note on the parasites. Contr Can Biol Fish 21:445–454
Hecky RE, Hesslein RH (1995) Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. J N Am Benthol Soc 14:631–653
Hecky RE, Smith REH, Barton DR, Guildford SJ, Taylor WD, Charlton MN, Howell T (2004) The nearshore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Can J Fish Aquat Sci 61:1285–1293
Henderson BA, Fry FEJ (1987) Interspecific relations among fish species in South Bay, Lake Huron, 1949–84. Can J Fish Aquat Sci 44:10–14
Hobson KA (1990) Stable isotope analysis of marbled murrelets—evidence for fresh-water feeding and determination of trophic level. Condor 92:897–903
Hodell DA, Schelske CL (1998) Production, sedimentation, and isotopic composition of organic matter in Lake Ontario. Limnol Oceanogr 43:200–214
Ihssen PE, Evans DO, Christie WJ, Reckahn JA, Desjardine RL (1981) Life-history, morphology, and electrophoretic characteristics of five allopatric stocks of lake whitefish (Coregonus clupeaformis) in the Great Lakes region. Can J Fish Aquat Sci 38:1790–1807
Jensen OP, Benson BJ, Magnuson JJ, Card VM, Futter MN, Soranno PA, Stewart KM (2007) Spatial analysis of ice phenology trends across the Laurentian Great Lakes region during a recent warming period. Limnol Oceanogr 52:2013–2026
Jobling M (1981) Temperature tolerance and the final preferendum—rapid methods for the assessment of optimum growth temperatures. J Fish Biol 19:439–455
Johannsson OE, Dermott R, Graham DM, Dahl JA, Millard ES, Myles DD, LeBlanc J (2000) Benthic and pelagic secondary production in Lake Erie after the invasion of Dreissena spp. with implications for fish production. J Gt Lakes Res 26:31–54
Kelly MH, Hagar WG, Jardine TD, Cunjak RA (2006) Non-lethal sampling of sunfish and slimy sculpin for stable isotope analysis: how scale and fin tissue compare with muscle tissue. North Am J Fish Manage 26:921–925
King JR, Shuter BJ, Zimmerman AP (1997) The response of the thermal stratification of South Bay (Lake Huron) to climatic variability. Can J Fish Aquat Sci 54:1873–1882
Kinnunen RE (2003) Great Lakes commercial fisheries. Michigan Sea Grant. http://www.miseagrant.umich.edu/fisheries/fish-commercial.html. Accessed 1-1-2008
Lumb CE (2005) Comparison of lake whitefish (Coregonus clupeaformis) growth in Lake Erie and Lake Ontario. M.Sc. thesis, University of Windsor, Windsor
Lumb CE, Johnson TB, Cook HA, Hoye JA (2007) Comparison of lake whitefish (Coregonus clupeaformis) growth, condition, and energy density between lakes Erie and Ontario. J Gt Lakes Res 33:314–325
McNickle GG, Rennie MD, Sprules WG (2006) Changes in benthic invertebrate communities of South Bay, Lake Huron following invasion by zebra mussels (Dreissena polymorpha), and potential effects on lake whitefish (Coregonus clupeaformis) diet and growth. J Gt Lakes Res 32:180–193
Mills KH, Chalanchuk SM (2004) The fin-ray method of aging lake whitefish. Ann Zool Fenn 41:215–223
Mohr LC, Ebener MP (2005) Status of lake whitefish (Coregonus clupeaformis) in Lake Huron. In: Mohr LC, Nalepa TF (eds) Proceedings of a workshop on the dynamics of lake whitefish (Coregonus clupeaformis) and the amphipod Diporeia spp. in the Great Lakes. Great Lakes Fishery Commission Technical Report 66, pp 105–126
Nalepa TF, Hartson DJ, Fanslow DL, Lang GA, Lozano SJ (1998) Declines in benthic macroinvertebrate populations in southern Lake Michigan, 1980–1993. Can J Fish Aquat Sci 55:2402–2413
Nalepa TF, Fanslow DL, Iii AJF, Lang GA, Eadie BJ, Quigley MA (2006) Continued disappearance of the benthic amphipod Diporeia spp. in Lake Michigan: is there evidence for food limitation? Can J Fish Aquat Sci 63:872–890
Nalepa TF, Fanslow DL, Pothoven SA, Foley AJ, Lang GA (2007) Long-term trends in benthic macroinvertebrate populations in Lake Huron over the past four decades. J Gt Lakes Res 33:421–436
Owens RW, Dittman DE (2003) Shifts in the diets of slimy sculpin (Cottus cognatus) and lake whitefish (Coregonus clupeaformis) in Lake Ontario following the collapse of the burrowing amphipod Diporeia. Aquat Ecosys Health Manage 6:311–323
Perga ME, Gerdeaux D (2003) Using the δ13C and δ15N of whitefish scales for retrospective ecological studies: changes in isotope signatures during the restoration of Lake Geneva, 1980–2001. J Fish Biol 63:1197–1207
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Pothoven SA, Madenjian CP (2008) Changes in consumption by alewives and lake whitefish after dreissenid mussel invasions in Lakes Michigan and Huron. North Am J Fish Manage 28:308–320
Pothoven SA, Nalepa TF, Schneeberger PJ, Brandt SB (2001) Changes in diet and body condition of lake whitefish in southern Lake Michigan associated with changes in benthos. North Am J Fish Manage 21:876–883
R Development Core Team (2006) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Reckahn JA (1970) Ecology of young lake whitefish (Coregonus clupeaformis) in South Bay, Manitoulin Island, Lake Huron. In: Lindsay CC, Woods CS (eds) The biology of coregonid fishes. University of Manitoba Press, Winnipeg, pp 437–460
Sierszen ME, Peterson GS, Scharold JV (2006) Depth-specific patterns in benthic-planktonic food web relationships in Lake Superior. Can J Fish Aquat Sci 63:1496–1503
Sinnatamby RN, Bowman JE, Dempson JB, Power M (2007) An assessment of de-calcification procedures for δ13C and δ15N analysis of yellow perch, walleye and Atlantic salmon scales. J Fish Biol 70:1630–1635
Smith BR, Tibbles JJ (1980) Sea lamprey (Petromyzon marinus) In lakes Huron, Michigan, and Superior - history of invasion and control, 1936–78. Can J Fish Aquat Sci 37:1780–1801
Suess HE (1955) Radiocarbon concentration in modern wood. Science 122:415–417
Syvaranta J, Vesala S, Rask M, Ruuhijarvi J, Jones RI (2008) Evaluating the utility of stable isotope analyses of archived freshwater sample materials. Hydrobiologia 600:121–130
Vander Zanden MJ, Rasmussen JB (1999) Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80:1395–1404
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Verburg P (2007) The need to correct for the Suess effect in the application of δ13C in sediment of autotrophic Lake Tanganyika, as a productivity proxy in the Anthropocene. J Paleolimnol 37:591–602
Wang Q, An SQ, Ma ZJ, Zhao B, Chen JK, Li B (2006) Invasive Spartina alterniflora: biology, ecology and management. Acta Phytotaxon Sin 44:559–588
Zar JH (1999) Biostatistical analysis. Prentice Hall, Toronto
Acknowledgments
Chesley West helped prepare lake whitefish tissues for isotopic analysis, and Randolph Fernandez and Michael Yuille sorted benthic invertebrate samples. Nina Jakobi and Bridget Dilauro sorted and identified stomach contents for contemporary whitefish samples. John Stinchcombe graciously provided access to his microbalance. Bill Mark, Mike Power, Jake Vander Zanden, Chelsey Lumb and Blake Matthews provided insights into sample preparation and study design. Tanya Kenesky and Andrew Nicholson helped prepare ESM S1. Dave Anderson provided advice on interpreting archived data codes. Luke Hillyer, Nina Jakobi and Rob Keetch and the past and present captain and crew of the Atygamayg provided field support. Thanks to Bryan Henderson for reviving the South Bay field program in 2001. Insightful comments from Bob Hecky improved the quality of the manuscript. This work was supported financially by grants from the Ontario Ministry of Natural Resources and the Canada Ontario Agreement to WGS, Natural Sciences and Engineering Research Council of Canada grants to MDR and WGS, a research grant from the Toronto Sportsmen’s Show and the Ontario Federation of Anglers and Hunters to MDR, Ontario Graduate Scholarships to MDR, and a Norman S. Baldwin Fishery Science Scholarship to MDR. The experiments performed here comply with the current laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dag Olav Hesseb.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rennie, M.D., Sprules, W.G. & Johnson, T.B. Resource switching in fish following a major food web disruption. Oecologia 159, 789–802 (2009). https://doi.org/10.1007/s00442-008-1271-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1271-z