Abstract
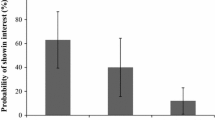
The majority of herbivorous insects have relatively specialized food habits. This suggests that specialization has some advantage(s) over generalization. Traditionally, feeding specialization has been thought to be linked to digestive or other food-related physiological advantages, but recent theory suggests that generalist natural enemies of herbivorous insects can also provide a major selective pressure for restricted host plant range. The European swallowtail butterfly Papilio machaon utilizes various plants in the Apiaceae family as hosts, but is an ecological specialist being monophagous on Angelica archangelica in southern Sweden. This perennial monocarp grows in three seaside habitat types: (1) on the barren rocky shore in the absence of any surrounding vegetation, (2) on the rocky shore with some surrounding vegetation, and (3) on species-rich meadows. The rocky shore habitat harbors few invertebrate generalist predators, whereas a number of invertebrate predators abound in the meadowland habitat. Here, we test the importance of enemy-free space for feeding specialization in Papilio machaon by assessing survival of larvae placed by hand on A. archangelica in each of the three habitat types, and by assessing the habitat-specificity of adult female egg-laying behavior by recording the distribution of eggs laid by free-flying adult females among the three habitat types. Larval survival was substantially higher in the rocky shore habitat than in the meadowland and significantly higher on host plants without surrounding vegetation on the rocky shore. Eggs laid by free-flying females were found in all three habitat types, but were significantly more frequent in the rocky shore habitat, suggesting that females prefer to lay eggs in the habitat type where offspring survival is highest. These results show that larval survivorship on the same host plant species can be strongly habitat-specific, and suggest that enemy-free space is an underlying factor that drives feeding specialization in Papilio machaon.



Similar content being viewed by others
References
Bernays E, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Blau WS (1980) The effect of environmental disturbance on a tropical butterfly population. Ecology 61:1005–1012
Damman H, Feeny P (1988) Mechanisms and consequences of selective oviposition by the zebra swallowtail butterfly. Anim Behav 36:563–573
Doak P, Kareiva P, Kingsolver J (2006) Fitness consequences of choosy oviposition for a time-limited butterfly. Ecology 87:395–408
Feeny P (1976) Plant apparency and chemical defense. In: Wallace J, Mansell R (eds) Recent Advances in Phytochemistry, 10. Plenum Press, New York, pp 1–40
Feeny P, Blau WS, Kareiva PM (1985) Larval growth and survivorship of the black swallowtail in central New York. Ecology 55:167–187
Forsberg J (1987) Size discrimination among conspecific hostplants in two pierid butterflies, Pieris napi and Pontia daplidice. Oecologia 72:52–57
Friberg M, Olofsson M, Berger D, Karlsson B, Wiklund C (2008) Habitat choice precedes host plant choice–niche separation in a species pair of a generalist and a specialist butterfly. Oikos (in press) doi:10.1111/j.2008.0030–1299.16740.x
Gehan EA (1965a) A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 52:203–223
Gehan EA (1965b) A generalized two-sample Wilcoxon test for doubly censored data. Biometrika 52:650–653
Gilbert LE (1979) Development of theory in insect-plant interactions. In: Horn DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. Ohio State University Press, Columbus, pp 117–154
Grossmueller DW, Lederhouse RC (1985) Oviposition site selection: an aid to rapid growth and development in the tiger swallowtail butterfly, Papilio glaucus. Oecologia 66:68–73
Kuussaari M, van Nouhuys S, Hellmann JJ, Singer MC (2004) Larval biology of checkerspots. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a model system for population biology. Oxford University Press, Oxford, pp 138–160
Murphy SM (2004) Enemy-free space maintains swallowtail butterfly host shift. Proc Natl Acad Sci USA 101:18048–18052
Parmesan C (1991) Evidence against “plant apparency” as a constraint on evolution of insect search efficiency. J Insect Behav 4:417–430
Pyke DA, Thompson JN (1986) Statistical analysis of survival and removal rate experiments. Ecology 67:240–245
Singer MC (1971) Evolution of food-plant preference in the butterfly Euphydryas editha. Evolution 25:383–389
Singer MC, Lee JR (2000) Discrimination within and between host species by a butterfly: implications for design of preference experiments. Ecol Lett 3:101–105
Singer MC, Thomas CD, Billington HL, Parmesan C (1994) Correlates of speed of evolution of host preferences in a set of twelve populations of the butterfly Euphydryas editha. Ecoscience 1:107–114
StatSoft (2007) STATISTICA (data analysis software system) version 8.0. www.statsoft.com. StatSoft, Tulsa
Tiritilli ME, Thompson JN (1988) Variation in swallowtail/plant interactions: host selection and the shapes of survivorship curves. Oikos 53:153–160
Thomas CD, Singer MC (1998) Scale-dependent evolution of specialization in a checkerspot butterfly: from individuals to metapopulations and ecotypes. In: Mopper S, Strauss SY (eds) Genetic structure and local adaptation in natural insect populations. Chapman & Hall, New York, pp 343–374
Thompson JN (1988) Coevolution and alternative hypotheses on insect/plant interactions. Ecology 69:893–895
Thompson JN (1993) Preference hierarchies and the origin of geographic specialization in host use in swallowtail butterflies. Evolution 47:1585–1594
Thompson JN, Pellmyr O (1991) Evolution of oviposition behavior and host preference in Lepidoptera. Annu Rev Entomol 36:65–89
Watanabe M (1981) Population dynamics of the swallowtail butterfly, Papilio xuthus in a deforested area. Res Popul Ecol (Kyoto) 23:74–93
Wehling WF, Thompson JN (1997) Evolutionary conservatism of oviposition preference in a widespread polyphagous insect herbivore, Papilio zelicaon. Oecologia 111:209–215
Wiklund C (1974) The concept of oligophagy and the natural habitats and host plants of Papilio machaon in Fennoscandia. Entomol Scand 5:151–160
Wiklund C (1975) The evolutionary relationship between adult oviposition preferences and larval host plant range in Papilio machaon. Oecologia 18:185–197
Wiklund C (1982) Generalist versus specialist utilization of host plants among butterflies. In: Wisser JH, Minks AK (eds) Insect–plant relationships. PUDOC, Wageningen, pp 181–192
Wiklund C (1984) Egg-laying patterns in butterflies in relation to their phenology and the visual apparency and abundance of their host plants. Oecologia 63:23–29
Acknowledgements
Thanks to Torbjörn Kronestedt for determination of Enoplognatha ovata. This study was supported by a grant from the Swedish Research Council to C.W. The experiments comply with the current laws of Sweden.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Konrad Fiedler.
Rights and permissions
About this article
Cite this article
Wiklund, C., Friberg, M. Enemy-free space and habitat-specific host specialization in a butterfly. Oecologia 157, 287–294 (2008). https://doi.org/10.1007/s00442-008-1077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1077-z