Abstract
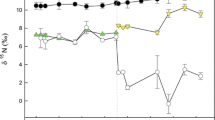
The use of stable isotopes to investigate animal diets, habitat use, and trophic level requires understanding the rate at which animals incorporate the 13C and 15N from their diets and the factors that determine the magnitude of the difference in isotopic composition between the animal’s diet and that of its tissues. We determined the contribution of growth and catabolic turnover to the rate of 13C and 15N incorporation into several tissues that can be sampled non-invasively (skin, scute, whole blood, red blood cells, and plasma solutes) in two age classes of a rapidly growing ectotherm (loggerhead turtles, Caretta caretta). We found significant differences in C and N incorporation rates and isotopic discrimination factors (Δ13C = δ13Ctissues − δ13Cdiet and Δ15N = δ15Ntissues − δ15Ndiet) among tissues and between age classes. Growth explained from 26 to 100% of the total rate of incorporation in hatchling turtles and from 15 to 52% of the total rate of incorporation in juvenile turtles. Because growth contributed significantly to the rate of isotopic incorporation, variation in rates among tissues was lower than reported in previous studies. The contribution of growth can homogenize the rate of isotopic incorporation and limit the application of stable isotopes to identify dietary changes at contrasting time scales and to determine the timing of diet shifts. The isotopic discrimination factor of nitrogen ranged from −0.64 to 1.77‰ in the turtles’ tissues. These values are lower than the commonly assumed average 3.4‰ discrimination factors reported for whole body and muscle isotopic analyses. The increasing reliance on non-invasive and non-destructive sampling in animal isotopic ecology requires that we recognize and understand why different tissues differ in isotopic discrimination factors.




Similar content being viewed by others
References
Adkins JN, Varnum SM, Auberry KJ, Moore KJ, Angell NH, Smith RD, Springer LD, Pounds JG (2002) Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics 1:947–955
Araujo MS, Bolnick DI, Machado G, Giaretta AA, dos Reis SF (2007) Using δ13C stable isotopes to quantify individual-level diet variation. Oecologia 152:643–654
Bösl C, Grupe A, Peters J (2006) A late Neolithic vertebrate food web based on stable isotope analyses. Int J Osteoarchaeol 16:296–315
Brown JH, West GB, Enquist BJ (2000) Scaling in biology: patterns, processes, causes, and consequences. In: Brown JH, West GB (eds) Scaling in biology. Oxford University Press, New York, pp 1–24
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. Springer, New York
Carleton SA, Martínez del Rio C (2005) The effect of cold-induced increased metabolic rate on the rate of 13C and 15N incorporation in house sparrows (Passer domesticus). Oecologia 114:226–232
Castanet J (1994) Age estimation and longevity in reptiles. Gerontology 40:174–192
Cerling TE, Harris JM (1999) Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleontological studies. Oecologia 120:347–363
Dalerum F, Angerbjorn A (2005) Resolving temporal variation in vertebrate diets using naturally occurring stable isotopes. Oecologia 144:647–658
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Dodds ED, McCoy MR, Geldenhuys A, Rea LD, Kennish JM (2004) Microscale recovery of total lipids from fish tissue by accelerated solvent extraction. J Am Oil Chem Soc 81:835–840
Fogel ML, Tuross N (2003) Extending the limits of paleodietary studies in humans with compound specific carbon isotope analysis of amino acids. J Archaeol Sci 30:535–545
Fry B, Arnold C (1982) Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 54:200–204
Gaye-Seisseggar J, Focken U, Abel H, Becker K (2003) Feeding level and diet quality influence trophic shift of C and N in Nile tilapia (Oreocromis niloticus (L.)). Isotopes Environ Health Stud 39:125–134
Gaye-Seisseggar J, Focken U, Muetzel S, Abel H, Becker K (2004) Feeding level and individual metabolic rate affect δ13C and δ15N values in carp: implications for food web studies. Oecologia 138:175–183
Gustafson L, Showers W, Kwak T, Levine J, Stoktof M (2007) Temporal and spatial variability in stable isotope compositions of a fresh water mussel: implications for biomonitoring and ecological studies. Oecologia 152:140–150
Haschemeyer AEV, Smith MAK (1979) Protein synthesis in liver, muscle, and gill of mullet (Mugil cephalus L.) in vivo. Biol Bull 156:93–102
Hesslein RH, Hallard KA, Ramal P (1993) Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can J Fish Aquat Sci 50:2071–2076
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188
Hobson KA, Alsaukas RT, Clark RG (1993) Stable nitrogen enrichment in avian tissues due to fasting and nutritional stress: implications for isotopic analysis of diet. Condor 95:388–394
Houlihan DF, Carter CG, McCarthy I (1995) Protein turnover in animals. In: Walsh PJ, Wright P (eds) Nitrogen metabolism and excretion. CRC Press, Boca Raton, pp 1–32
Howland MR, Corr LT, Young SMM, Jones V, Jim S, Van der Merwe NJ, Mitchell AD, Evershed RP (2003) Expression of the dietary isotope signal in the compound-specific δ13C values of pig bone lipids and amino acids. Int J Osteoarchaeol 13:54–65
Iverson JB (1984) Proportional skeletal mass in turtles. Fla Sci 47:1–11
Kelly JF (1999) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Kohler A (1964) Variation in the growth of Atlantic cod (Gadus morhua L.). J Fish Res Bd Can 21:57–100
Kraemer JE, Bennett SH (1981) Utilization of posthatching yolk in loggerhead sea turtles, Caretta caretta. Copeia 1981:406–411
Lesage V, Hammill MO, Kovacs KM (2002) Diet-tissue fractionation of stable carbon and nitrogen in phocid seals. Mar Mamm Sci 18:182–193
MacAvoy SE, Macko SA, Garman GC (2001) Isotopic turnover in aquatic predators: quantifying the exploitation of migratory prey. Can J Fish Aquat Sci 56:923–932
MacAvoy SE, Macko SA, Arneson LS (2005) Growth versus metabolic tissue replacement in mouse tissues determined by stable carbon and nitrogen isotope analysis. Can J Zool 83:631–641
Martínez del Rio C, Wolf BO (2005) Mass balance models for animal isotopic ecology. In: Starck MA, Wang T (eds) Physiological and ecological adaptations to feeding in vertebrates. Science Publishers, Enfield, pp 141–174
McClelland JW, Montoya JP (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83:2173–2180
McCutchan JH Jr, Lewis WM Jr, Kendall C, McGraith CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
McIntyre PB, Flecker AS (2006) Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia 148:12–21
Michener RH, Schell DM (1994) Stable isotope ratios as tracers in marine aquatic food webs. In: Lathja K, Michener RH (eds) Stable isotopes in ecology and environmental science. Blackwell, New York, pp 138–157
Miller K, Birchard GF (2005) Influence of body size on shell mass in the ornate turtle, Terrapene ornate. J Herpetol 39:158–161
Newsome SD, Martínez del Rio C, Phillips DL, Bearhop S (2007) A niche for isotopic ecology. Front Ecol Environ 5:429–436
O’Brien DM, Schrag DP, Martínez del Rio C (2000) Allocation to reproduction in a hawkmoth: a quantitative analysis using stable isotopes. Ecology 81:2822–2831
Pearson SF, Levey DJ, Greenberg CH, Martínez del Rio C (2003) Effects of elemental composition on the incorporation of dietary nitrogen and carbon isotopic signatures in an omnivorous songbird. Oecologia 135:516–523
Perga ME, Gerdeaux D (2005) “Are fish what they eat” all year round? Oecologia 144:598–606
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Ann Rev Ecol Syst 18:293–320
Phillips DL, Eldridge PM (2006) Estimating the timing of diet shifts using stable isotopes. Oecologia 14:195–203
Pinnegar JK, Polunin NVC (1999) Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Funct Ecol 13:225–231
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Roth JD, Hobson KA (2000) Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive red fox: implications for dietary reconstruction. Can J Zool 78:848–852
Schoeller DA (1999) Isotopic fractionation: why aren’t we what we eat? J Archaeol Sci 26:667–673
Sebens KP (1987) The ecology of indeterminate growth in animals. Annu Rev Ecol Syst 18:371–407
Seminoff JA, Jones TT, Eguchi T, Jones DR, Dutton PH (2006) Stable isotope discrimination (δ13C and δ15N) between soft tissues of the green sea turtle Chelonia mydas and its diet. Mar Ecol Prog Ser 308:271–278
Stuart A, Ord JK (1994) Kendall’s advanced theory of statistics. Volume 1, distribution theory. Edward Arnold, New York
Sullivan JC, Buscetta KJ, Michener RH, Whitaker JO, Finnerty JR, Kunz TH (2006) Models developed from delta C-13 and delta N-15 of skin tissue indicate non-specific habitat use by the big brown bat (Eptesicus fuscus). Ecoscience 13:11–22
Swingle WM, Warmolts DI, Keinath JA, Musick JA (2005) Exceptional growth rates of captive loggerhead turtles, Caretta caretta. Zoo Biol 12:491–497
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37
Tominga O, Uno N, Seikai T (2003) Influence of diet shift from formulated feed to live mysids on the carbon and nitrogen stable isotope ratio δ13C and δ15N in dorsal muscles of juvenile Japanese flounders, Paralichthys olivaceus. Aquaculture 218:265–276
Tsudaka K, Moriyama T, Lieberman I (1971) Liver amino acid pool may be homogeneous with respect to protein synthesis. J Biochem 70:173–174
Turner MW, Hulme B (1970) The plasma proteins: an introduction. Pitman Medical & Scientific Publishing Company, London
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182
Wallace BP, Seminoff JA, Kilham SS, Spotila JR, Dutton PH (2006) Leatherback turtles as oceanographic indicators: stable isotope analyses reveal a trophic dichotomy between ocean basins. Mar Biol 149:953–960
West GB, Brown JH, Enquist BJ (2001) A general model for ontogenetic growth. Nature 413:628–631
Youngson AF, McLaren IS, Bacon PJ, Jones PJ, Jones W (2005) Seasonal growth patterns of wild juvenile fish: partitioning variation among explanatory variables based on individual growth trajectories. J Anim Ecol 74:1–11
Zimmerman DL, Núñez-Anton V, Gregoire TG, Schanenberger O, Hart JD, Kenward MG, Molenberghs G, Verbeke G, Pourahmadi M, Vieu P (2001) Parametric modelling of growth curve data: an overview. TEST 10:1–73
Acknowledgments
Without the insights and assistance of A. B. Bolten, this study would not have been possible. The authors thank L. Chapman, D. Hodell, and B. MacFadden for their assistance and support with this project and F. Davis, P. Eliazar, T. Garcia, H. Jacobson, S. Luciano, N. Osman, J. Pfaller, J. Sankar for their assistance with the logistics of turtle care and sample collection and preparation. We thank the University of Florida, Geological Sciences, Light Stable Isotope Lab and J. Curtis for assistance with stable isotope analyses. We also thank C. Layman for his helpful comments on this manuscript. This project was funded by Disney Wildlife Conservation Fund, National Marine Fisheries Service, US Fish and Wildlife Service, and Florida Fish and Wildlife Conservation Commission (FWC) Marine Turtle Grants Program and was conducted under permits issued by FWC (TP# 016) and the University of Florida Institutional Animal Care and Use Committee (permit Z094). C. Martínez del Rio was supported by NSF grant ISBN (0421738).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Craig Osenberg.
Rights and permissions
About this article
Cite this article
Reich, K.J., Bjorndal, K.A. & Martínez del Rio, C. Effects of growth and tissue type on the kinetics of 13C and 15N incorporation in a rapidly growing ectotherm. Oecologia 155, 651–663 (2008). https://doi.org/10.1007/s00442-007-0949-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0949-y