Abstract
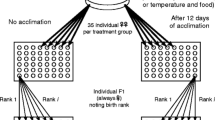
Life history theory is concerned with the costs of survival, growth and reproduction under different ecological conditions and the allocation of resources to meet these costs. Typical approaches used to address these topics include manipulation of food resources, followed by measures of subsequent reproductive traits, and measures of the relationship between current and future reproductive investment. Rarely, however, do studies test for the interaction of past investment, present resource availability and future investment simultaneously. Here, we investigate this interaction in females of a sexual parasite–host system consisting of the hybridogenetic frog Rana esculenta (E) and one of its parental species Rana lessonae (L). We kept females from each of two groups (with or without previous reproduction) under two food treatments (low or high) and regularly recorded their growth as well as their body condition and hormone titres as measures of future reproductive condition. After keeping them in hibernation until the following spring, we exposed the females to males, recorded whether they spawned or not and related this response to their condition in the previous autumn. Past reproduction negatively affected growth during summer and condition during autumn which, in turn, reduced the following year’s reproductive output. These costs of previous reproduction were less pronounced under the high than under the low food treatment and lower in R. lessonae than in R. esculenta. Increasing food supply improved reproductive condition more in L than in E females. These species differences in reproductive costs and food requirements provide a mechanistic explanation for why E females skip annual reproduction almost twice as often as L females. Since R. esculenta is a sexual parasite that depends on R. lessonae for successful reproduction, these species-specific life history patterns not only affect individual fitness but also the spatial structure and temporal dynamics of mixed LE populations.


Similar content being viewed by others
References
Abt G, Reyer H-U (2004) Larval development and recruitment of juveniles in a natural population of Rana lessonae and Rana esculenta. Copeia 2004:638–646
Anholt BR, Hotz H, Guex G-D, Semlitsch RD (2003) Overwinter survival of Rana lessonae and its hemiclonal associate Rana esculenta. Ecology 84:391–397
Banks PB, Powell F (2004) Does maternal condition or predation risk influence small mammal population dynamics? Oikos 106:176–184
Beckerman A, Benton TG, Ranta E, Kaitala V, Lundberg P (2002) Population dynamic consequences of delayed life-history effects. Trends Ecol Evol 17:263–269
Benton TG, Plaistow SJ, Beckerman AP, Lapsley CT, Littlejohns S (2005) Changes in maternal investment in eggs can affect population dynamics. Proc R Soc Lond B 272:1351–1356
Berger L (1977) Systematics and hybridization in the Rana esculenta complex. In: Taylor DH, Guttmann SI (eds) The reproductive biology of amphibians. Plenum, New York, pp 367–388
Berger L, Uzzell T (1980) The eggs of European water frogs (Rana esculenta complex) and their hybrids. Folia Biol (Kraków) 28:3-25
Berger L, Rybacki M, Hotz H (1994) Artificial fertilization of water frogs. Amphib Reptil 15:408-413
Bize P, Roulin A, Tella JL, Bersier LF, Richner H (2004) Additive effects of ectoparasites over reproductive attempts in the long-lived alpine swift. J Anim Ecol 73:1080–1088
Blankenhorn HJ (1977) Reproduction and mating behavior in Rana lessonae–Rana esculenta mixed populations. In: Taylor DH, Guttmann SI (eds) The reproductive biology of amphibians. Plenum, New York, pp 389–410
Brodmann PA, Reyer H-U (1999) Nestling provisioning in water pipits (Anthus spinoletta): do parents go for specific nutrients or profitable prey? Oecologia 120:506–514
Brown CA (2003) Offspring size-number trade-offs in scorpions: an empirical test of the Van Noordwijk and de Jong model. Evolution 57:2184–2190
Camargo A, Naya DE, Canavero A, da Rosa I, Maneyro R (2005) Seasonal activity and the body size-fecundity relationship in a population of Physalaemus gracilis (Boulenger, 1883) (Anura, Leptodactylidae) from Uruguay. Ann Zool Fenn 42:513–521
Castellano S, Cucco M, Giacoma C (2004) Reproductive investment of female green toads (Bufo viridis). Copeia 2004:659–664
Clutton-Brock T, Guiness FE, Albon SD (1983) The costs of reproduction to red deer hinds. J Anim Ecol 52:367–383
Covas R, Doutrleant C, du Plessis MA (2004) Experimental evidence of a link between breeding conditions and the decision to breed or help in a colonial cooperative bird. Proc R Soc Lond B 271:827–832
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 80:225–252
Elmberg J (1991) Ovarian cyclicity and fecundity in boreal common frogs Rana temporaria L. along a climatic gradient. Funct Ecol 5:340–350
Erelli MC, Elkinton JS (2000) Maternal effects on gypsy moth (Lepidoptera: Lymantriidae) population dynamics: a field experiment. Environ Entomol 29:476–488
Gatten RE Jr, Miller K, Full RJ (1992) Energetics at rest and locomotion. In: Feder ME, Burggren WW (eds) Environmental physiology of amphibians. The University of Chicago Press, Chicago, Ill., pp 314–377
Gignac A, Gregory PT (2005) The effects of body size, age, and food intake during pregnancy on reproductive traits of a viviparous snake, Thamnophis ordinoides. Ecoscience 12:236–243
Girish S, Saidapur SK (2000) Interrelationship between food availability, fat body, and ovarian cycles in the frog, Rana tigrina, with a discussion on the role of fat body in anuran reproduction. J Exp Zool 286:487–493
Graf J-D, Polls Pelaz (1989) Evolutionary genetics of the Rana esculenta complex. In: Dawley RM, Bogart, JP (eds) Evolution and ecology of unisexual vertebrates. New York State Mus Bull 466:289–302
Green AJ (2001) Mass/length residuals: measures of body condition or generators of spurious results? Ecology 82:1473-1483
Günther R (1990) Die Wasserfrösche Europas. Ziemsen, Wittenberg
Guex G-D, Hotz H, Semlitsch RD (2002) Deleterious alleles and differential viability in progeny of natural hemiclonal frogs. Evolution 56:1036–1044
Hellriegel B, Reyer H-U (2000) Factors influencing the composition of mixed populations of a hemiclonal hybrid and its sexual host. J Evol Biol 13:906–918
Holenweg Peter A-K (2001) Survival in adults of the water frog Rana lessonae and its hybridogenetic associate Rana esculenta. Can J Zool 79:652–661
Holenweg A-K, Reyer H-U (2000) Hibernating behavior of Rana lessonae and R. esculenta in their natural habitat. Oecologia 123:41–47
Holenweg Peter A-K, Reyer H-U, Abt Tietje G (2002) Ecological factors affecting genotype and sex ratios in mixed populations of Rana lessonae, R. ridibunda and their hybridogenetic associate R. esculenta. Ecoscience 9:1–11
Huber H, During HJ (2000) No long-term costs of meristem allocation to flowering in stolonferous Trifolium species. Evol Ecol 14:731–748
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61-67
Jørgensen CB (1986) Effect of fat body excision in female Bufo bufo on ipsilateral ovary, with a discussion of fat body–gonad relationships. Acta Zool 67:5-10
Koivula M, Koskela E, Mappes T, Oksanen TA (2003) Cost of reproduction in the wild: manipulation of reproductive effort in the bank vole. Ecology 84:398–405
Lampo M, Medialdea V (1996) Energy allocation patterns in Bufo marinus from two habitats in Venezuela. J Trop Ecol 12:321–331
Lardner B, Loman J (2003) Growth or reproduction? Resource allocation by female frogs Rana temporaria. Oecologia 137:541–546
Lengagne T, Grolet O, Joly P (2006) Male mating speed promote hybridization in the Rana lessonae–Rana esculenta waterfrog system. Behav Ecol Sociobiol 60:123–130
Lessells CM (1991) The evolution of life histories. In: Krebs JR, Davies NB (eds) Behavioural ecology, 3rd edn. Blackwell, Oxford, pp 32–68
Licht P, Mc Creery BR, Barnes R, Pang R (1983) Seasonal and stress related changes in plasma gonadotropins, sex steroids, and corticosterone in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol 50:124-145
Lüddecke H (1997) Field reproductive potential of tropical high mountain Hyla labialis females: direct and indirect evidence from mark–recapture data. Amphib Reptil 18:357–368
Nager RG, Ruegger C, Van Noordwijk AJ (1997) Nutrient or energy limitation on egg formation: a feeding experiment in great tits. J Anim Ecol 66:495–507
Neveu A (1991) Structures démographiques de populations adultes de grenouilles vertes du complexe R. esculenta. Bull Fr Peche Oiscicult 321:55–71
Pagano A, Joly P (1999) Limits of the morphometric method for field identification of water frogs. Alytes 16:130–138
Paolucci M, Esposito V, Difiore MM, Botte V (1990) Effects of short postcapture confinement on plasma reproductive hormone and corticosterone profiles in Rana esculenta during the sexual cycle. Boll Zool 57:253-259
Parejo D, Danchin E (2006) Brood size manipulation affects frequency of second clutches in the blue tit. Behav Ecol Sociobiol 60:184–194
Pough FH, Magnussen WE, Ryan MJ Wells KD, Taigen TL (1992) Behavioral energetics. In: Feder ME, Burggren WW (eds) Environmental physiology of amphibians. The University of Chicago Press, Chicago, Ill., pp 395–436
Redshaw MR (1972) The hormonal control of the amphibian ovary. Am Zool 12:289-306
Reyer H-U, Bättig B (2004) Identification of reproductive status in female frogs—a quantitative comparison of nine methods. Herpetologica 60:349–357
Reyer H-U, Frei G, Som C (1999) Cryptic female choice: frogs reduce clutch size when amplexed by undesired males. Proc R Soc Lond B 266:2101-2107
Reyer H-U, Wälti M-O, Bättig I, Altwegg R, Hellriegel B (2004) Low proportions of reproducing hemiclonal females increase the stability of a sexual parasite–host system (Rana esculenta, R. lessonae). J Anim Ecol 73:1089–1101
Ritke ME, Lessman CA (1994) Longitudinal-study of ovarian dynamics in female gray treefrogs (Hyla chrysoscelis). Copeia 1994:1014–1022
Roff DA (2002) Life history evolution. Sinauer, Sunderland, Mass.
Schmidt-Nielsen K (1983) Animal physiology: adaptation and environment, 3rd edn. Cambridge University Press, Cambridge
Schultz RJ (1969) Hybridization, unisexuality, and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am Nat 103:605-619
Sinsch U (1983) Wasserhaushalt und tagesperiodisches Verhalten von drei europäischen Rana-Arten (Amphibia: Anura). PhD thesis, University of Köln, Germany
Sinsch U (1992) Two new tagging methods for individual identification of amphibians in long-term field studies: first experiences with natterjacks. Salamandra 28:116-128
Snedecor GW, Cochran WG (1980) Statistical methods, 15th edn. Iowa State University Press, Ames, Iowa
Som C, Anholt BR, Reyer H-U (2000) The effect of assortative mating on the coexistence of a hybridogenetic waterfrog and its sexual host. Am Nat 156:34-46
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Svensson E (1995) Avian reproductive timing—when should parents be prudent? Anim Behav 49:2569–1575
Uller T, Olsson M (2005) Trade-offs between offspring size and number in the lizard Lacerta vivipara: a comparison between field and laboratory conditions. J Zool 265:295–299
Uzzell T, Berger L (1975) Electrophoretic phenotypes of Rana ridibunda, Rana lessonae and their hybridogenetic associate, Rana esculenta. Proc Acad Nat Sci Philadelphia 127:13-24
Van der Kraak G, Lin HR, Donaldson EM, Dye HM, Hunter GA (1983) Effects of LH-RH and des-Gly10(D-Ala6)LH-RH-ethylamide on plasma gonadotropin levels and oocyte maturation in adult female coho salmon (Oncorhynchus kisutch). Gen Comp Endocrinol 40:470–476
Van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources—their influence on variation in life-history tactics. Am Nat 128:137–142
Vorburger C (2001) Fixation of deleterious mutations in clonal lineages: evidence from hybridogenetic frogs. Evolution 55:2319–2332
Acknowledgements
We are grateful to the numerous people who helped us catch frogs and to our colleagues I. Bättig (University of Zürich) and I. Zerenner-Fritsche (University of Bayreuth, Germany) for conducting the testosterone analyses from blood samples and to S. Röthlisberger for performing the enzyme analysis. We also thank two anonymous reviewers for their valuable comments on a previous version of the manuscript. The study was part of a project funded by the Swiss National Science Foundation through a grant to H.-U. Reyer (no. 31-40688.94). Catching of frogs, keeping them in captivity and all methods applied in the experiment conform to the ethical and animal care guidelines issued by the Swiss Academy of Natural Sciences and were granted by the Veterinary Office of the Canton Zurich (permit nos. 133/96 and 131/97).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Anssi Laurila.
Rights and permissions
About this article
Cite this article
Waelti, M.O., Reyer, HU. Food supply modifies the trade-off between past and future reproduction in a sexual parasite–host system (Rana esculenta, Rana lessonae). Oecologia 152, 415–424 (2007). https://doi.org/10.1007/s00442-007-0671-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0671-9