Abstract
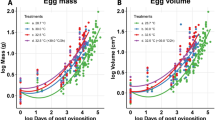
Cold environmental temperature is detrimental to reproduction by oviparous squamate reptiles by prolonging incubation period, negatively affecting embryonic developmental processes, and by killing embryos in eggs directly. Because low soil temperature may prevent successful development of embryos in eggs in nests, the geographic distributions of oviparous species may be influenced by the thermal requirements of embryos. In the present study, we tested the hypothesis that low incubation temperature determines the northern distributional limit of the oviparous lizard Sceloporus undulatus. To compare the effects of incubation temperature on incubation length, egg and hatchling survival, and hatchling phenotypic traits, we incubated eggs of S. undulatus under temperature treatments that simulated the thermal environment that eggs would experience if located in nests within their geographic range at 37°N and north of the species’ present geographic range at latitudes of 44 and 42°N. After hatching, snout–vent length (SVL), mass, tail length, body condition (SVL relative to mass), locomotor performance, and growth rate were measured for each hatchling. Hatchlings were released at a field site to evaluate growth and survival under natural conditions. Incubation at temperatures simulating those of nests at 44°N prolonged incubation and resulted in hatchlings with shorter SVL relative to mass, shorter tails, shorter hind limb span, slower growth, and lower survival than hatchlings from eggs incubated at temperatures simulating those of nests at 37 and 42°N. We also evaluated the association between environmental temperature and the northern distribution of S. undulatus. We predicted that the northernmost distributional limit of S. undulatus would be associated with locations that provide the minimum heat sum (∼495 degree-days) required to complete embryonic development. Based on air and soil temperatures, the predicted northern latitudinal limit of S. undulatus would lie at ∼40.5–41.5°N. Our predicted value closely corresponds to the observed latitudinal limit in the eastern United States of ∼40°N. Our results suggest that soil temperatures at northern latitudes are not warm enough for a sufficient length of time to permit successful incubation of S. undulatus embryos. These results are consistent with the hypothesis that incubation temperature is an important factor limiting the geographic distributions of oviparous reptile species at high latitudes and elevations.




Similar content being viewed by others
References
Andrews RM (1982) Patterns of growth in reptiles. In: Gans C, Pough FH (eds) Biology of the Reptilia, vol 13. Wiley, New York, pp 273–320
Andrews RM (2000) Evolution of viviparity in squamate reptiles (Sceloporus spp): a variant of the cold-climate model. J Zool 250:243–253
Andrews RM, Qualls CP, Rose BR (1997) Effects of low temperature on embryonic development of Sceloporus lizards. Copeia 1997:827–833
Andrews RM, Mathies T, Warner DA (2000) Effects of incubation temperature on morphology, growth, and survival of juvenile Sceloporus undulatus. Herpetol Monogr 14:420–431
Angilletta MJ, Winters RS, Dunham AE (2000) Thermal effects on the energetics of lizard embryos: implications for hatchling phenotypes. Ecology 81:2957–2968
Beerling DJ (1993) Impact of temperature on the northern distribution limits of the introduced species of Fallopia japonica and Impatiens glandulifera in North-West Europe. J Biogeogr 20:45–56
Blouin-Demers G, Weatherhead PJ, Row JR (2004) Phenotypic consequences of nest-site selection in black rat snakes (Elaphe obsoleta). Can J Zool 82:449–456
Bobyn ML, Brooks RJ (1994) Incubation conditions as potential factors limiting the northern distribution of snapping turtles, Chelydra serpentina. Can J Zool 72:28–37
Braña F (2003) Morphological correlates of burst speed and field movement patterns: the behavioral adjustment of locomotion in wall lizards (Podarcis muralis). Biol J Linn Soc 80:135–146
Burger J (1991) Effects of incubation temperature on behavior of hatchling pine snakes: implications for reptilian distribution. Behav Ecol Sociobiol 28:297–303
Dufaure J, Hubert J (1961) Table de development du lezard vivipare: Lacerta (Zootica) vivipara Jaquin. Arch Anat Microsc Morphol Exp 50:309–327
Elphick MJ, Shine R (1998) Long-term effects of incubation temperatures on the morphology and locomotor performance of hatchling lizards Bassiana duperreyi, Scincidae). Biol J Linn Soc 63:429–447
Ferguson GW, Fox SF (1984) Annual variation of survival advantage of large juvenile side-blotched lizards, Uta stansburiana: its causes and evolutionary significance. Evolution 38:342–349
Greer AE (1968) Modes of reproduction in the squamate faunas of three altitudinally correlated life zones in east Africa. Herpetologica 24:229–232
Hecnar SJ (1994) Nest site distribution, site selection, and brooding in the five-lined skink (Eumeces fasciatus). Can J Zool 72:1510–1151
Irschick DJ (2000) Effects of behavior and ontogeny on the locomotor performance of a West Indian lizard: Anolis lineatopus. Funct Ecol 14:438–444
Irschick DJ (2003) Measuring performance in nature: implications for studies of fitness within populations. Integr Comp Biol 43:396–407
Janzen DH (1967) Why mountain passes are higher in the tropics. Am Nat 101:233–249
Kearney M, Porter WP (2004) Mapping the fundamental niche: physiology, climate, and the distribution of a nocturnal lizard. Ecology 11:3119–3131
Kolbe JJ, Losos LB (2005) Hind limb plasticity in Anolis carolinensis. J Herpetol 39:674–678
Litzgus JD, Brooks RJ (1998) Reproduction in a northern population of Clemmys guttata. J Herpetol 32:252–259
Losos JB, Creer DA, Glossip D, Goellner R, Hampton A, Roberts G, Haskell N, Taylor P, Ettling J (2000) Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54:301–305
McGovern GM, Knisley CB (1986) Prey selection experiments and predator–prey size relationships in eastern fence lizards, Sceloporus undulatus hyacinthynus from Virginia. Va J Sci 37:9–15
Méndez-de la Cruz FR, Villagrán-Santa Cruz M, Andrews RM (1998) Evolution of viviparity in the lizard genus Sceloporus. Herpetologica 54:521–532
Muth A (1980) Physiological ecology of desert iguana (Dipsosaurus dorsalis) eggs: temperature and water relations. Ecology 61:1335–1343
National Climatic Data Center (2005) United States Department of Commerce, http://www.ncdc.noaa.gov/oa/ncdc.html (Cited 2 January 2005)
Natural Resources Conservation Service and National Water and Climate Center (2005) United State Department of Agriculture, http://www.wcc.nrcs.usda.gov (Cited 15 May 2005)
Packard GC, Tracy CR, Roth JJ (1977) The physiological ecology of reptilian eggs and embryos and the evolution of viviparity within the class Reptilia. Biol Rev 52:71–105
Parker SL, Andrews RM, Mathies TM (2004) Embryonic responses to variation in oviductal oxygen in the lizard Sceloporus undulatus from New Jersey and South Carolina, USA. Biol J Linn Soc 83:289–299
Porter WP, Sabo JL, Tracy CR, Reichman OJ, Ramankutty N (2002) Physiology on a landscape scale: plant animal interactions. Integr Comp Biol 42:431–453
Qualls CP, Andrews RM (1999) Cold climates and the evolution of viviparity in reptiles: cold incubation temperatures produce poor-quality offspring in the lizard Sceloporus virgatus. Biol J Linn Soc 67:477–491
Qualls FJ, Shine R (1998) Geographic variation in lizard phenotypes: importance of the incubation environment. Biol J Linn Soc 64:477–491
Qualls FJ, Shine R (2000) Post hatching environment contributes greatly to phenotypic variation between two populations of the Australian garden skink Lampropholis guichenoti. Biol J Linn Soc 71:315–341
SAS Institute (2003) SAS version 9.1.2. SAS Institute, Cary, N.C.
Sergeev AM (1940) Researches on the viviparity in reptiles. Mosc Soc Nat (Jubilee Issue):1–34
Sexton OJ, Marion KR (1974) Duration of incubation of Sceloporus undulatus eggs at constant temperature. Physiol Zool 47:91–95
Shine R (1983) Reptilian viviparity in cold climates: testing the assumptions of an evolutionary hypothesis. Oecologia 57:397–405
Shine R (1985) The evolution of viviparity in reptile: an ecological analysis. In: Gans C, Billett F (eds) Biology of the Reptilia, vol 15. Wiley, New York, pp 605–694
Shine R (1987) Reproductive mode may determine geographic distributions in Australian venomous snakes (Pseudechis, Eliapidae). Oecologia 71:608–612
Shine R (1997) Seasonal timing of oviposition in sand lizards (Lacerta agilis): why earlier clutches are better. J Evol Biol 10:369–381
Shine (1999) Egg-laying reptiles in cold climates: determinants and consequences of nest temperatures in montane lizards. J Evol Biol 12:918–926
Shine R (2002) Reconstructing an adaptationist scenario: what selective forces favor the evolution of viviparity in montane reptiles? Am Nat 160:582–593
Shine R (2004) Incubation regimes of cold-climate reptiles: the thermal consequences of nest-site choice, viviparity and maternal basking. Biol J Linn Soc 83:145–155
Shine R, Berry JF (1978) Climatic correlates of live-bearing in squamate reptiles. Oecologia 33:261–268
Shine R, Bull JJ (1979) The evolution of live-bearing in lizards and snakes. Am Nat 113:905–923
Shine R, Madsen TR, Elphick MJ, Harlow PS (1997) The influence of nest temperatures and maternal brooding on hatchling phenotypes in water pythons. Ecology 78:1713–1721
Sites JW Jr, Archie JW, Cole CJ, Villela OF (1992) A review of the phylogenetic hypotheses for lizards of the genus Sceloporus (Phrynosomatidae): implications for ecological and evolutionary studies. Bull Am Mus Nat Hist 213:1–110
St. Clair RC, Gregory PT (1991) Factors affecting the northern range limit of painted turtles (Chrysemys picta): winter acidosis or freezing? Copeia 1990:1083–1089
Stebbins RC (1985) A field guide to western reptiles and amphibians, 2nd edn. Houghton Mifflin, Boston, Mass.
Tinkle DW, Ballinger RE (1972) Sceloporus undulatus: a study of the intraspecific demography of a lizard. Ecology 53:570–574
Tinkle DW, Gibbons JW (1977) The distribution and evolution of viviparity in reptiles. Misc Publ Mus Zool Univ Mich 154:1–55
Travis JM, McManus MG, Baer CF (1999) Sources of variation in physiological phenotypes and their evolutionary significance. Am Zool 39:422–433
Ungerer MJ, Ayres MP, Lombardero MJ (1999) Climate and the northern distribution limits of Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). J Biogeogr 26:1113–1145
Warner DA, Andrews RM (2002) Laboratory and field experiments identify the sources of variation in phenotypes and survival of hatchling lizards. Biol J Linn Soc 76:105–124
Weekes HC (1933) On the distribution, habitat and reproductive habits of certain European and Australian snakes and lizards, with particular regard to their adoption of viviparity. Proc Linn Soc NSW 58:270–274
Wilson BS (1991) Latitudinal variation in activity season mortality rates of the lizard Uta stansburiana. Ecol Monogr 61:393–414
Yntema CL (1978) Incubation times for eggs of the turtle Chelydra serpentina (Testudines: Chelydridae) at various temperatures. Herpetologica 34:274–277
Zalom FG, Goodell PB, Wilson LT, Barnett WW, Bently WJ (1983) Degree-days: the calculation and use of heat units in pest management. Leaflet 21373. Division of Agriculture and Natural Resources, University of California Berkeley, Berkeley, Calif.
Acknowledgements
Funding was provided by a grant from the Virginia Academy of Sciences awarded to S. L. P. We thank Mr and Mrs L. Flowers for permission to conduct fieldwork on their property, M. Denbow, T. Jenssen, R. Jones, A. McNabb, S. Adolph and an anonymous reviewer for their comments on the manuscript, J. Barthet for assistance with graphics software, and M. Barthet and H. Schwend for assistance in the field. Experimental and animal maintenance protocols were approved by the Virginia Tech Animal Care Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mark Chappell.
Rights and permissions
About this article
Cite this article
Parker, S.L., Andrews, R.M. Incubation temperature and phenotypic traits of Sceloporus undulatus: implications for the northern limits of distribution. Oecologia 151, 218–231 (2007). https://doi.org/10.1007/s00442-006-0583-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0583-0