Abstract
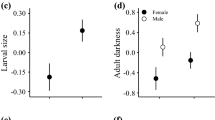
Body size is a major component of fitness. However, the relative contributions of different factors to optimal size, and the determinants of spatial and temporal variation in size, have not been fully established empirically. Here, we use a mesocosm of a Drosophilidae assemblage inhabiting decaying nectarines to investigate the influence of spatial variation in temperature on adult body size in Drosophila simulans Sturtevant. Two treatments were established; one in the sun where developing larvae were exposed to high temperatures and the other in the shade where temperature conditions were milder. The simple developmental effects of temperature differences (i.e. larger flies are likely to emerge from cooler environments), or the simple effects of stressful temperatures (i.e. high temperatures yield wing abnormalities and smaller flies), were overridden by interactive effects between temperature and larval density. Emergences were lower in the sun than shade, probably as a result of temperature-induced mortality. However, flies attained the same final sizes in the shade and sun. In addition, abnormally winged flies were clustered in the shaded treatments. In the shade treatments, where emergences were higher than in the sun, stressful conditions as a result of high larval density likely resulted in wing abnormalities and small size. Consequently, there was little spatial variation in size across the mesocosm, but substantial spatial variation in abundance. Under natural conditions both mortality and non-lethal effects of temperature and/or crowding are likely to play a role in the evolution of body size.





Similar content being viewed by others
References
Al-Saffar ZY, Grainger JNR, Aldrich J (1995) Influence of constant and changing temperature and humidity on the development and survival of the eggs and pupae of Drosophila melanogaster (Meigen). J Therm Biol 20:389–397
Angilletta MJ, Steury TD, Sears MW (2004) Temperature, growth rate, and body size in ectotherms, fitting pieces of a life-history puzzle. Integr Comp Biol 44:498–509
Atkinson WD (1979) A field investigation of larval competition in domestic Drosophila. J Anim Ecol 48:91–102
Atkinson WD (1983) Gregarious oviposition in Drosophila melanogaster is explained by surface texture. Aust J Zool 31:925–929
Atkinson D (1994) Temperature and organism size—a biological law for ectotherms? Adv Ecol Res 25:1–58
Atkinson WD, Shorrocks B (1984) Aggregation of larval Diptera over discrete and ephemeral breeding sites: the implications for coexistence. Am Nat 124:336–351
Azevedo RBR, French V, Partridge L (2002) Temperature modulates epidermal cell size in Drosophila melanogaster. J Insect Physiol 48:231–237
Bangham J, Chapman T, Partridge L (2002) Effects of body size, accessory gland and testis size on pre- and postcopulatory success in Drosophila melanogaster. Anim Behav 64:915–921
Barker JSF (1983) Interspecific competition. In: Ashburner M, Carson HL, Thompson JN (eds) The genetics and biology of Drosophila. Academic Press, New York, pp 285–341
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small. Q Rev Biol 75:385–407
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol 44:413–424
Borash DJ, Gibbs AG, Joshi A, Mueller LD (1998) A genetic polymorphism maintained by natural selection in a temporally varying environment. Am Nat 151:148–156
Boulétreau J (1978) Ovarian activity and reproductive potential in a natural population of Drosophila melanogaster. Oecologia 35:319–342
Bubli OA, Imasheva AG, Loeschcke V (1998) Selection for knockdown resistance to heat in Drosophila melanogaster at high and low larval densities. Evolution 52:619–625
Chippindale AK, Chu TJF, Rose MR (1996) Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 50:753–766
Collet D (1991) Modelling binary data. Chapman and Hall, London
Cowley DE, Atchley WR (1990) Development and quantitative genetics of correlation structure among body parts of Drosophila melanogaster. Am Nat 135:242–268
Coyne JA, Beecham E (1987) Heritability of two morphological characters within and among natural populations of Drosophila melanogaster. Genetics 117:727–737
Dahlgaard J, Hasson E, Loeschcke V (2001) Behavioural differentiation in oviposition activity in Drosophila buzzatii from highland and lowland populations in Argentina: plasticity or thermal adaptation? Evolution 55:738–747
David JR, Gibert P, Gravot E, Pétavy G, Morin JP, Karan D, Moreteau B (1997) Phenotypic plasticity and developmental temperature in Drosophila: analysis and significance of reaction norms of morphometrical traits. J Therm Biol 22:441–451
Delcour J, Lints FA (1966) Environmental and genetic variations of wing size, cell size and cell division rate, in Drosophila melanogaster. Genetica 37:543–556
De Moed GH, De Jong G, Scharloo W (1997) Environmental effects on body size variation in Drosophila melanogaster and its cellular basis. Genet Res 70:35–43
Dobson AJ (2002) An introduction to generalized linear models. Chapman and Hall, CRC Texts in Statistical Science, Boca Raton, Fla.
Feder ME, Blair N, Figueras H (1997) Oviposition site selection: unresponsiveness of Drosophila to cues of potential thermal stress. Anim Behav 53:585–588
Feder ME, Krebs RA (1998) Natural and genetic engineering of the heat-shock protein Hsp70 in Drosophila melanogaster: consequences for thermotolerance. Am Zool 38:503–517
French V, Feast M, Partridge L (1998) Body size and cell size in Drosophila: the developmental response to temperature. J Insect Physiol 44:1081–1089
Gibbs AG, Chippindale AK, Rose MR (1997) Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J Exp Biol 200:1821–1832
Gibbs AG, Matzkin LM (2001) Evolution of water balance in the genus Drosophila. J Exp Biol 204:2331–2338
Hageman J, Eisses KT, Jacobs PJM, Scharloo W (1990) Ethanol in Drosophila cultures as a selective factor. Evolution 44:447–454
Heard SB, Remer LC (1997) Clutch-size behavior and coexistence in ephemeral-patch competition models. Am Nat 150:744–770
Hoffmann AA, Scott M, Partridge L, Hallas R (2003a) Overwintering in Drosophila melanogaster: outdoor field cage experiments on clinal and laboratory selected populations help to elucidate traits under selection. J Evol Biol 16:614–623
Hoffmann AA, Sørensen JG, Loeschcke V (2003b) Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol 28:175–216
Houle D, Rowe L (2003) Natural selection in a bottle. Am Nat 161:50–67
James AC, Partridge L (1995) Thermal evolution of rate of larval development in Drosophila melanogaster in laboratory and field populations. J Evol Biol 8:315–330
James AC, Partridge L (1998) Geographic variation in competitive ability in Drosophila melanogaster. Am Nat 151:530–537
Jenkins NL, Hoffmann AA (2000) Variation in morphological traits and trait asymmetry in field Drosophila serrata from marginal populations. J Evol Biol 13:113–130
Karan D, Parkash R (1998) Desiccation tolerance and starvation resistance exhibit opposite latitudinal clines in Indian geographical populations of Drosophila kikkawai. Ecol Entomol 23:391–396
Kari JS, Huey RB (2000) Size and seasonal temperature in free-ranging Drosophila subobscura. J Therm Biol 25:267–272
Korie S, Perry JN, Mugglestone MA, Clark SJ, Thomas CFG, Mohamad Roff MN (2000) Spatiotemporal associations in beetle and virus count data. J Agric Biol Environ Stat 5:214–239
Kozłowski J, Czarnołęski M, Dańko M (2004) Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr Comp Biol 44:480–493
Krijger CL, Peters YC, Sevenster JG (2001) Competitive ability of neotropical Drosophila predicted from larval developmental times. Oikos 92: 325–332
Legendre P, Fortin M-J (1989) Spatial patterning and ecological analysis. Vegetatio 80:107–138
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam
McCabe J, Partridge L (1997) An interaction between environmental temperature and genetic variation for body size for the fitness of adult female Drosophila melanogaster. Evolution 51:1164–1174
McCullagh P, Nelder JA (1989) Generalized linear models. 2nd edn. Chapman and Hall, CRC, Fla.
Morin JP, Moreteau B, Pétavy G, David JR (1999) Divergence of reaction norms of size characters between tropical and temperate populations of Drosophila melanogaster and D. simulans. J Evol Biol 12:329–339
Partridge L, Barrie B, Fowler K, French V (1994) Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48:1269–1276
Perry JN (1995) Spatial analysis by distance indices. J Anim Ecol 64:303–314
Perry JN, Bell ED, Smith RH, Woiwood IP (1996) SADIE: software to measure and model spatial patterns. Asp Appl Biol 46:95–102
Perry JN, Winder L, Holland JM, Alston RD (1999) Red-blue plots for detecting clusters in count data. Ecol Lett 2:106–113
Pétavy G, Moreteau B, Gibert P, Morin J-P, David JR (2001) Phenotypic plasticity of body size in Drosophila: effects of a daily periodicity of growth temperature in two sibling species. Physiol Entomol 26:351–361
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Multiple and complex regression. Cambridge University Press, UK, pp 111–154
Roberts SP, Feder ME (1999) Natural hyperthermia and expression of the heat shock protein Hsp70 affect developmental abnormalities in Drosophila melanogaster. Oecologia 121:323–329
Roff DA (2002) Life history evolution. Sinauer, Sunderland, Mass.
Rutherford SL, Lindquist S (1998) Hsp90 as a capacitor for morphological evolution. Nature 396:336–342
Santos M, Fowler K, Partridge L (1994) Gene–environment interaction for body size and larval density in Drosophila melanogaster: an investigation of effects on development time, thorax length and adult sex ratio. Heredity 72:515–521
Scheiring JF, Davis DG, Ranasinghe A, Teare CA (1984) Effects of larval crowding on life history parameters in Drosophila melanogaster Meigen (Diptera: Drosophilidae). Exp Gerontol 77:329–332
Sevenster JG, Van Alphen JM (1993) A life history trade-off in Drosophila species and community structure in variable environments. J Anim Ecol 62:720–736
Sokal RR, Wartenberg DE (1983) A test of spatial autocorrelation analysis using an isolation-by-distance model. Genetics 105:219–237
Srivastava DS, Kolasa J, Bengtsson J, Gonzalez A, Lawler SP, Miller TE, Munguia P, Romanuk T, Schneider DC, Trzcinski MK (2004) Are natural microcosms useful model systems for ecology? Trends Ecol Evol 19:379–384
Sørensen JG, Loeschcke V (2001) Larval crowding in Drosophila melanogaster induces Hsp70 expression, and leads to increased adult longevity and adult thermal stress resistance. J Insect Physiol 47:1301–1307
Thomas RH (1993) Ecology of body size in Drosophila buzzatii: untangling the effects of temperature and nutrition. Ecol Entomol 18:84–90
Unwin DM, Corbet SA (1991) Insects, plants and microclimate. Richmond, Surrey
Warren M, McGeoch MA, Chown SL (2003) Predicting abundance from occupancy: a test for an aggregated insect assemblage. J Anim Ecol 72:468–477
Wartenberg D (1989) SAAP v 4.3—a spatial autocorrelation analysis program. Robert Wood Johnson Medical School, Piscataway
Weeks AR, McKenchnie SW, Hoffmann AA (2002) Dissecting adaptive clinal variation: markers, inversion and size/stress associations in Drosophila melanogaster from a central field population. Ecol Lett 5:756–763
Wertheim B, Marchais J, Vet LEM, Dicke M (2002) Allee effect in larval resource exploitation in Drosophila: an interaction among density of adults, larvae, and micro-organisms. Ecol Entomol 27:608–617
Worthen WB, Hipp MN, Twardokus CT, Roller RR (1993) Effects of ant predation and larval density on mycophagous fly communities. Oikos 66:526–532
Zwaan BJ, Bijlma R, Hoekstra RF (1992) On the developmental theory of ageing. II. The effect of developmental temperature on longevity in relation to adult body size in D. melanogaster. Heredity 68:123–130
Acknowledgements
Nordi Fruits is thanked for supplying the nectarines. We thank Joe Perry for discussion of SADIE, and Ruan Veldtman for comments on an earlier draft of the manuscript. Two anonymous referees provided helpful and insightful comments on an earlier version of the manuscript. This work was supported by the National Research Foundation (GUN2053618) and by the Mellon Foundation Mentoring Scheme, University of Pretoria.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Hoffmeister
Rights and permissions
About this article
Cite this article
Warren, M., McGeoch, M.A., Nicolson, S.W. et al. Body size patterns in Drosophila inhabiting a mesocosm: interactive effects of spatial variation in temperature and abundance. Oecologia 149, 245–255 (2006). https://doi.org/10.1007/s00442-006-0434-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0434-z