Abstract
Trophic linkages across habitats are thought to be strong when areas of different productivity are juxtaposed. Reefs dominated by macroalgae are commonly juxtaposed to less productive seagrass beds. We tested if macroalgae detached from 12 rocky reefs in south-western Australia were exported to adjacent seagrass beds and consumed by seagrass-associated fauna. We also assessed the extent of linkages by testing for patterns in biomass and consumption of reef algae, and density of herbivorous fish with increasing distance away from reefs.
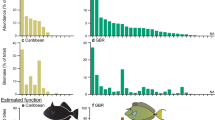
Detached reef algae were found in seagrass beds adjacent to all reefs. The biomass varied among reefs and with distance from reef, but detached reef algae within the seagrass beds comprised up to 23% (mean 3.6% ± 0.7 SE) of attached algae growing on an equivalent area of reef. Maximum accumulations were found immediately adjacent to reefs (0 m) and at the furthest distance away (>300 m). Kelp (Ecklonia radiata) dominated the attached and detached algae, and up to 77% of the biomass of E. radiata tethered in seagrass beds were consumed over 5 days (mean 11.7% ± 0.5 SE). There were more herbivorous fish at 0 m than at >300 m away from reefs, and consumption of tethered kelp was typically highest at 0 m, but was in some cases highest at >300 m.
Our study documents that, over hundreds of kilometres of coastline, macroalgae are exported from reefs to adjacent seagrass beds where they are consumed by seagrass-associated fauna. While reef algae in seagrass beds may be a patchy resource at a single time, at landscape scales and over longer time periods, the supply will be relatively predictable. We therefore suggest that detached reef algae form a significant trophic link between reefs and seagrass beds, and that this trophic link extends to distances of at least hundreds of metres away from individual reefs.




Similar content being viewed by others
References
Bell SS, Hall MO, Robbins BD (1995) Toward a landscape approach in seagrass beds: using macroalgal accumulation to address questions of scale. Oecologia 104:163–168
Bustamante RH, Branch GM, Eekhout S (1995) Maintenance of an exceptional intertidal grazer biomass in South Africa: subsidy by subtidal kelps. Ecology 76:2314–2329
Cambridge ML, Hocking PJ (1997) Annual primary production and nutrient dynamics of the seagrasses Posidonia sinuosa and Posidonia australis in south-western Australia. Aquat Bot 59:277–295
Cebrian J (1999) Patterns in the fate of production in plant communities. Am Nat 154:449–468
Cebrian J (2004) Role of first-order consumers in ecosystem carbon flow. Ecol Lett 7:232–240
Eggleston DB, Grover JJ, Lipcius RN (1988) Ontogenetic diet shifts in Nassau Groper: trophic linkages and predatory impact. Bull Mar Sci 63:111–126
Gambi MC, Zupo V, Buia MC, Mazzella L (2000) Feeding ecology of Platynereis dumerilii (Auduin & Milne-Edwards) in the seagrass Posidonia oceanica system: the role of the epiphytic flora (Polychaeta, Nereidae). Ophelia 53:189–202
Giller PS, Hillebrand H, Berninger U-G, Gessner MO, Hawkins SJ, Inchausti P, Inglis C, Leslie H, Malmqvist B, Monaghan MT, Morin PJ, O’Mullan G (2004) Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. Oikos 104:423–436
Goldberg NA, Kendrick GA (2004) Effects of island groups, depth, and exposure to ocean waves on subtidal macroalgal assemblages in the Recherche Archipelago, Western Australia. J Phycol 40:631–641
Granata TC, Serra T, Colomer J, Casamitjana X, Duarte CM, Gacia E (2001) Flow and particle distributions in a nearshore seagrass meadow before and after a storm. Mar Ecol Prog Ser 218:95–106
Harman N, Harvey ES, Kendrick GA (2003) Differences in fish assemblages from different reef habitats at Hamelin Bay, south-western Australia. Mar Freshw Res 54:177–184
Hawkins SJ (2004) Scaling up: the role of species and habitat patches in functioning of coastal ecosystems. Aquatic Conserv. Mar Freshw Ecosyst 14:217–219
Hill NA, Blount C, Poore AGB, Worthington D, Steinberg PD (2003) Grazing effects of the sea urchin Centrostephanus rodgersii in two contrasting rocky reef habitats: effects of urchin density and its implications for the fishery. Mar Freshw Res 54:691–700
Howard RK (1989) The structure of a nearshore fish community of Western Australia: diel patterns and the habitat role of limestone reefs. Environ Biol Fish 24:93–104
Hyndes GA, Kendrick AJ, MacArthur LD, Stewart E (2003) Differences in the species- and size-composition of fish assemblages in three distinct seagrass habitats with differing plant and meadow structure. Mar Biol 142:1195–1206
Kendrick GA (1999) Western Australia. In: Andrew N (ed) Under southern seas—the ecology of Australia’s rocky reefs. University of New South Wales Press Ltd., Sydney pp 50–57
Kendrick GA, Harvey E, Wernberg T, Harman N, Goldberg N (2004) The role of disturbance in maintaining diversity of benthic macroalgal assemblages in southwestern Australia. Jap J Phycol (Sorui) 52:5–9
Kennelly SJ (1987) Physical disturbances in an Australian kelp community. I. Temporal effects. Mar Ecol Prog Ser 40:145–153
Kirkman H, Kendrick GA (1997) Ecological significance and commercial harvesting of drifting and beach-cast macro-algae and seagrasses in Australia: a review. J App Phycol 9:311–326
Kirkman H, Walker DI (1989) Regional studies - Western Australian seagrass. In: Larkum AWD, McComb AJ, Shepherd S (eds) Biology of seagrasses—a treatise on the biology of seagrasses with special reference to the Australian region. Elsevier, Amsterdam pp 157–181
Klumpp DW, Howard RK, Pollard DA (1989) Trophodynamics and nutritional ecology of seagrass communities. In: Larkum AWD, McComb AJ, Shepherd S (eds) Biology of seagrasses—a treatise on the biology of seagrasses with special reference to the Australian region. Elsevier, Amsterdam pp 394–457
Lavery PS, Vanderklift MA (2002) A comparison of spatial and temporal patterns in epiphytic macroalgal assemblages of the seagrasses Amphibolis griffithii and Posidonia coriacea. Mar Ecol Prog Ser 236:99–112
Lemm AJ, Hegge BJ, Masselink G (1999) Offshore wave climate, Perth (Western Australia), 1994–96. Mar Freshw Res 50:95–102
Mann KH (1973) Seaweeds: their productivity and strategy for growth. Science 182:975–981
Masselink G, Pattiaratchi CB (2001) Characteristics of the sea breeze system in Perth, Western Australia, and its effect on the nearshore wave climate. J Coastal Res 17:173–187
Meyer JL, Schultz ET (1985) Migrating haemulid fishes as a source of nutrients and organic matter on coral reefs. Limnol Oceanogr 30:146–156
Montgomery WL, Gerkin SD (1980) Marine macroalgae as foods for fishes: an evaluation of potential food quality. Environ Biol Fish 5:143–153
Moran D, Clements KD (2002) Diet and endogenous carbohydrases in the temperate marine herbivorous fish Kyphosus sydneyanus. J Fish Biol 60:1190–1203
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci 98:166–170
Norderhaug KM, Fredriksen S, Nygaard K (2003) Trophic importance of Laminaria hyperborea to kelp forest consumers and the importance of bacterial degradation to food quality. Mar Ecol Prog Ser 255:135–144
Phillips JC, Kendrick GA, Lavery PS (1997) A test of a functional group approach to detecting shifts in macroalgal communities along a disturbance gradient. Mar Ecol Prog Ser 153:125–138
Polis GA, Hurd SD (1995) Extraordinarily high spider densities on islands: flow of energy from the marine to terrestrial food webs and the absence of predation. Proc Natl Acad Sci 92:4382–4386
Polis GA, Hurd SD (1996) Linking marine and terrestrial food webs; allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am Nat 147:396–423
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Ann Rev Ecol Syst 28:289–316
Randall JE (1965) Grazing effect on sea grasses by herbivorous reef fishes in the West Indies. Ecology 46:255–260
Rimmer DW, Wiebe WJ (1987) Fermentative microbial digestion in herbivorous fishes. J Fish Biol 31:229–236
Robertson AI, Lucas JS (1983) Food choice, feeding rates, and the turnover of macrophyte biomass by a surf-zone inhabiting amphipod. J Exp Mar Biol Ecol 72:99–124
Searle DJ, Semeniuk V (1985) The natural sectors of the inner Rottnest Shelf coast adjoining the Swan Coastal Plain. J R Soc WA 67:116–136
Seymour RJ, Tegner MJ, Dayton PK, Parnell PE (1989) Storm wave induced mortality of giant kelp, Macrocystis pyrifera, in southern California. Est Coast Shelf Sci 28:277–292
Steinberg PD (1995) Interaction between the canopy dwelling echinoid Holopneustes purpurescens and its host kelp Ecklonia radiata. Mar Ecol Prog Ser 127:169–181
Thomsen MS, Wernberg T (2005) Mini review: what affects the forces required to break or dislodge macroalgae? Eur J Phycol 40:1–10
Thomsen MS, Wernberg T, Kendrick GA (2004) The effect of thallus size, life stage, aggregation, wave exposure and substrate conditions on the forces required to break or dislodge the small kelp Ecklonia radiata. Bot Mar 47:454–460
Vanderklift MA, Lavery PS (2000) Patchiness in assemblages of epiphytic macroalgae on Posidonia coriacea at a hierarchy of spatial scales. Mar Ecol Prog Ser 192:127–135
Wernberg T, Kendrick GA, Phillips JC (2003) Regional differences in kelp-associated algal assemblages on temperate limestone reefs in south-western Australia. Divers Distrib 9:427–441
Acknowledgements
This research was supported by the Australian Research Council, the Department of Environmental Protection (Western Australia) and Edith Cowan University. We thank Lachlan MacArthur, Tim Daly, Cameron Sim, Kirsten Wiseman, Andrew Tennyson and Matt Kletzcowski for assistance in the field. Phillip England provided insightful comments on the manuscript. The work detailed in this paper comply with the laws of Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christian Koerner
Rights and permissions
About this article
Cite this article
Wernberg, T., Vanderklift, M.A., How, J. et al. Export of detached macroalgae from reefs to adjacent seagrass beds. Oecologia 147, 692–701 (2006). https://doi.org/10.1007/s00442-005-0318-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0318-7