Abstract
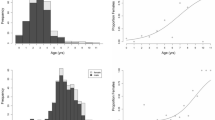
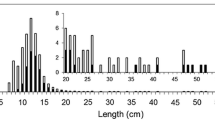
The ultimate body size that an individual fish achieves can be a function both of direct effects of growth or indirect effects associated with the timing of sexual maturation (and associated energetic tradeoffs). These alternatives are often invoked to explain variation in body size within and among fish populations, but have rarely been considered simultaneously. We assessed how resource availability and timing of maturation interact to influence individual body size of bluegill (Lepomis macrochirus). Resource availability (high and low food) and the social structure of the population (presence or absence of large, mature males) were varied in experimental ponds. Food ration affected growth (larger fish in the high food treatments) and the social structure of the population affected timing of maturation (early maturation of males in the absence of large males). Treatment effects, however, were sex-specific; males responded to the social structure of the population and females were more responsive to resource availability. We also found individuals that became sexually mature were smaller than those that remained immature, although results were sex-specific and resource dependent. For males, individuals that matured were smaller when resources were limited; mature and immature females showed no difference in body size regardless of food ration. We show that both resource availability and the processes that control timing of maturation interact in sex-specific ways to influence body size of bluegill. These results suggest that a more robust explanation for variable body size requires consideration of sex-specific interactions between ecological (food and growth) and evolutionary (timing of maturation) mechanisms.



Similar content being viewed by others
References
Abrahams MV, Dill LM (1989) A determination of the energetic equivalence of the risk of predation. Ecology 70:999–1007
Aday DD, Kush CM, Wahl DH, Philipp DP (2002) The influence of stunted body size on the reproductive ecology of bluegill Lepomis macrochirus. Ecol Freshwat Fish 11:190–195
Aday DD, Wahl DH, Philipp DP (2003) Assessing population-specific and environmental influences on bluegill life histories: a common garden approach. Ecology 84:3370–3375
Amundsen PM, Klemetsen A (1988) Diet, gastric evacuation rates and food consumption in a stunted population of Arctic charr, Salvelinus alpinus L., in Takvatn, northern Norway. J Fish Biol 33:697–709
Bateman AJ (1948) Intrasexual selection in Drosophila. Heredity 2:349–368
Beard TD, Essington TE (2000) Effects of angling and life history processes on bluegill size structure: insights from an individual-based model. Trans Am Fish Soc 129:561–568
Belk MC (1993) Growth and mortality of juvenile sunfishes (Lepomis sp.) under heavy predation. Ecol Freshwat Fish 2:91–98
Bell G (1980) The costs of reproduction and their consequences. Am Nat 116:45–76
Borowsky RL (1978) Social inhibition in natural populations of Xiphophorus variatus (Pisces: Poeciliidae). Science 201:933–935
Borowsky RL (1987) Agonistic behavior and social inhibition of maturation in fishes of the genus Xiphophorus (Poeciliidae). Copeia 1987:792–796
Boyd CE, Lichtkoppler F (1979) Water quality management in pond fish culture. Research and Development Series 22. Auburn University, Auburn, Alabama
Claussen JC (1991) Annual variation in the reproductive activity of a bluegill population: effect of clutch size and temperature. Master’s Thesis, University of Toronto, Toronto
Coble DW (1988) Effects of angling on bluegill populations: management implications. N Am J Fish Man 8:277–283
Damsgard B, Langeland A (1994) Effects of stocking piscivorous brown trout, Salmo trutta, L. on stunted Arctic charr, Salvelinus alpinus, L. Ecol Freshwat Fish 3:59–66
Danylchuk AJ, Fox MG (1994) Seasonal reproductive patterns of pumpkinseed (Lepomis gibbosus) with varying body size characteristics. Can J Fish Aquat Sci 51:490–500
Dettmers JM, Stein RA (1992) Food consumption by larval gizzard shad: zooplankton effects and implications for reservoir communities. Trans Am Fish Soc 121:494–507
Diana JS (1987) Simulation of mechanisms causing stunting in Northern pike populations. Trans Am Fish Soc 116:612–617
Donald DB, Alger DJ (1986) Stunted lake trout (Salvelinus namaycush) from the Rocky mountains. Can J Fish Aquat Sci 43:608–612
Fletcher DA, Wootton RJ (1995) A hierarchical response to differences in ration size in the reproductive performance of female three-spined sticklebacks. J Fish Biol 46:657–668
Fox MG (1994) Growth, density and interspecific influences on pumpkinseed life histories. Ecology 75:1157–1171
Fox MG, Crivelli AJ (1998) Body size and reproductive allocation in a multiple spawning centrarchid. Can J Fish Aquat Sci 55:737–748
Gadgil M, Bossert WJ (1970) Life historical consequences of natural selection. Am Nat 104:1–24
Gross MR (1982) Sneakers, satellites, and parentals: polymorphic mating strategies in North American sunfishes. Z Tierpsychol 60:1–26
Gross MR, MacMillan AM (1981) Predation and the evolution of colonial nesting in bluegill sunfish (Lepomis macrochirus). Behav Ecol Sociobiol 8:163–174
Hirshfield MF (1980) An experimental analysis of reproductive effort and cost in the Japanese Medaka Oryzias latipes. Ecology 61:282–292
Jansen WA (1996) Plasticity in maturity and fecundity of yellow perch, Perca flavescens (Mitchill): comparisons of stunted and normal-growing populations. Ann Zool Fennici 33:403–415
Jennings MJ, Claussen JE, Philipp DP (1997) Effect of population size structure on reproductive investment of male bluegill. N Am J Fish Man 17:516–524
Justus JA, Fox MG (1994) The cost of early maturation on growth, body condition, and somatic lipid content in a lake pumpkinseed (Lepomis gibbosus) population. Ecol Freshwat Fish 3:9–17
Konkle BR, Sprules WG (1986) Planktivory by stunted lake trout in an Ontario lake. Trans Am Fish Soc 115:515–521
Mann GJ, McCart PJ (1981) Comparison of sympatric normal and dwarf populations of least cisco (Coregonus sardinella) inhabiting Trout Lake, Yukon Territory. Can J Fish Aquat Sci 38:240–244
Mittelbach GG, Osenburg CW (1993) Stage-structured interactions in bluegill: consequences of adult resource variation. Ecology 74:2381–2394
Morita K, and Morita SH (2002) Rule of age and size at maturity: individual variation in the maturation history of resident white-spot charr. J Fish Biol 61:1230–1238
Otis KJ, Piette RR, Keppler JE, Rasmussen PW (1998) A largemouth bass fishery to control an overabundant bluegill population in a Wisconsin lake. J Freshwat Ecol 13:391–403
Persson L (1983) Food consumption and competition between age classes in a perch Perca fluviatilis population in a shallow eutrophic lake. Oikos 40:197–207
Ricker WE (1981) Changes in the average size and average age of Pacific salmon. Can J Fish Aquat Sci 38:1636–1656
Ridgway LL, Chapleau F (1994) Study of a stunted population of yellow perch (Perca flavescens) in a monospecific lake in Gatineau Park, Quebec. Can J Zool 72:1576–1582
Roff DA (1983) An allocation model of growth and reproduction in fish. Can J Fish Aquat Sci 40:1395–1404
Roff DA (1984) The evolution of life history parameters in teleosts. Can J Fish Aquat Sci 41:989–1000
Santucci VJ Jr, Wahl DH (2003) The effects of growth, predation, and first-winter mortality on recruitment of bluegill cohorts. Trans Am Fish Soc 132:346–360
Schaffer WF, Elson PF (1975) The adaptive significance of variations in life history among local populations of Atlantic salmon in North America. Ecology 56:577–590
Sohn JJ (1977) Socially induced inhibition of genetically determined maturation in the platyfish, Xiphophorus maculatus. Science 195:199–201
Stearns SC (1976) Life history tactics: a review of the ideas. Q Rev Biol 51:3–47
Stearns SC (1992) The evolution of life histories. Oxford University Press
Stearns SC, Koella JC (1986) The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution 40:893–913
Swingle HS, Smith EV (1941) The management of ponds with stunted fish populations. Trans Am Fish Soc 71:102–105
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine, Chicago, pp 136–179
Welker MT, Pierce CL, Wahl DH (1994) Growth and survival of larval fishes: roles of competition and zooplankton abundance. Trans Am Fish Soc 124:703–717
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Ann Rev Ecol Syst 15:393–425
Whiteman HH (1997) Maintenance of polymorphism promoted by sex-specific fitness payoffs. Evolution 51:2039–2044
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690
Winemiller KO, Rose KA (1992) Patterns of life-history diversification in North American fishes: implications for population regulation. Can J Fish Aquat Sci 49:2196–2218
Ylikarjula J, Heino M, Dieckmann U (1999) Ecology and adaptation of stunted growth in fish. Evol Ecol 13:433–453
Acknowledgments
We are grateful for the assistance of T. Jaecks, B. Braetigam, E. Ozier, K. Schnake, and B. Davis for assistance with sampling and processing. Earlier drafts of this manuscript were improved by suggestions from Drs. R. Fischer, S. Robinson, R. Warner, N. Metcalfe, E. Marschall, the Aquatic Ecology Discussion Group at the Kaskaskia Biological Station, and an anonymous reviewer. Statistical advice was provided by the University of Illinois Statistical Consulting Service. Funding for this project was provided in part by the Federal Aid in Sportfish Restoration Act, Project F-128-R administered by the Illinois Department of Natural Resources (IDNR). We thank M. Conlin, L. Dunham, S. Stuewe, and S. Pallo for coordinating activities with the IDNR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Libby Marschall
Rights and permissions
About this article
Cite this article
Aday, D.D., Philipp, D.P. & Wahl, D.H. Sex-specific life history patterns in bluegill (Lepomis macrochirus): interacting mechanisms influence individual body size. Oecologia 147, 31–38 (2006). https://doi.org/10.1007/s00442-005-0242-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0242-x