Abstract
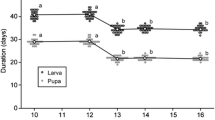
The oviposition-preference–offspring-performance hypothesis predicts that female insects should prefer to deposit clutches on or in hosts that maximize offspring performance. An important assumption behind this prediction is that female fitness is tightly correlated with the fitness of any one offspring. In this study, we evaluate offspring performance in the walnut fly, Rhagoletis juglandis Cresson (Diptera: Tephritidae), in relation to a previously described oviposition preference for previously exploited host fruit. In particular, we examined how superparasitism of walnut hosts influences offspring survival and weight at pupation under field conditions. We found that superparasitism was common and that increases in larval densities within fruit were associated with reduced larval survival and weight at pupation. In a laboratory experiment, female size was correlated with lifetime fecundity. In this system, oviposition preference is therefore negatively, not positively, correlated with offspring performance. We argue that patterns of female preference in this system reflect direct benefits to females that are traded off against costs in terms of offspring fitness. Because female fitness is a product not only of offspring quality but also of the total number of offspring produced, female walnut flies may be optimizing their fitness by producing many less fecund offspring. Studies examining the preference-performance hypothesis should consider the reproductive conflicts between parents and offspring as potential factors that influence the congruence between parental preference and offspring performance.



Similar content being viewed by others
References
van Alphen JJM, Visser ME (1990) Superparasitism as an adaptive strategy for insect parasitoids. Annu Rev Entomol 35:59–79
van Alphen JJM, Visser ME, Nell HW (1992) Adaptive superparasitism and patch time allocation in solitary parasitoids: searching in groups vs. sequential patch visits. Funct Ecol 6:528–535
Averill AL, Prokopy RJ (1987) Intraspecific competition in the tephritid fruit fly Rhagoletis pomonella. Ecology 68:878–886
Ballabeni P, Wlodarczyk M, Rahier M (2001) Does enemy-free space for eggs contribute to a leaf beetle’s oviposition preference for a nutritionally inferior host plant? Funct Ecol 15:318–324
Bernays EA (2001) Neural limitations in phytophagous insects: implications for diet breadth and evolution of host affiliation. Annu Rev Entomol 46:703–727
Bonduriansky R, Brooks RJ (1999) Reproductive allocation and reproductive ecology of seven species of Diptera. Ecol Entomol 24b:389–395
Burk T, Webb JC (1983) Effect of male size on calling propensity, song parameters, and mating success in Caribbean fruit flies, Anestrepha suspensa (Loew) (Diptera, Tiphritidae). Ann Entomol Soc Am 76:678–682
Bush GL (1966) The taxonomy, cytology and evolution of the genus Rhagoletis in North America (Diptera: Tephritidae). Bull Mus Comp Zool Harv Univ 134:431–562
Charnov EL, Skinner SW (1985) Complementary approaches to the understanding of parasitoid oviposition decisions. Environ Entomol 14:383–391
Copp NH, Davenport D (1978) Agraulis and passiflora. I. control of specificity. Biol Bull 155:98–112
Courtney SP, Kibota TT (1990) Mother doesn’t know best: selection of host by ovipositing insects. In: Bernays EA (ed) Insect-plant interactions. CRC, Boca Raton, Fla., pp 161–188
Craig TP, Itami JK, Price PW (1989) A strong relationship between oviposition preference and larval performance in a shoot-galling sawfly. Ecology 70:1691–1699
Credland PF, Dick KM, Wright AW (1986) Relationship between larval density, adult size and egg production in the cowpea seed beetle, Callosobruchus maculatus. Ecol Entomol 11:41–50
Denno RF, Larsson S, Olmstead KL (1990) Role of enemy-free space and plant quality in host–plant selection by willow beetles. Ecology 71:124–137
Einum S, Fleming IA (2000) Highly fecund mothers sacrifice offspring survival to maximize fitness. Nature 405:565–567
Etges WJ, Heed WB (1987) Sensitivity to larval density in populations of Drosophila mojavensis: influences of host plant variation on components of fitness. Oecologia 71:375–381
Forbes LS (1991) Optimal size and number of offspring in a variable environment. J Theor Biol 150:299–304
Fox CW (1993) A quantitative genetic analysis of oviposition preference and larval performance on two hosts in the bruchid beetle, Callosobruchus maculatus. Evolution 47:166–175
Godfray HCJ (1987) The evolution of clutch size in parasitic wasps. Am Nat 129:221–233
Howard DJ, Bush GL (1989) Influence of bacteria on larval survival and development in Rhagoletis (Diptera: Tephritidae). Ann Entomol Soc Am 82:633–640
Howard DJ, Bush GL, Breznak JA (1985) The evolutionary significance of bacteria associated with Rhagoletis. Evolution 39:405–417
Jaenike J (1978) Optimal oviposition behavior in phytophagous insects. Theor Popul Biol 14:350–356
Karban R, Courtney S (1987) Intraspecific host plant choice: lack of consequences for Streptanthus tortuosus (Cruciferae) and Euchloe hyantis (Lepidoptera: Pieridae). Oikos 48:243–248
Lalonde RG, Mangel M (1994) Seasonal effects on superparasitism by Rhagoletis completa. J Anim Ecol 63:583–588
Landolt PJ, Averill AL (1999) Fruit Flies. In: Hardie J, Minks AK (eds) Pheromones of non-lepidopteran insects associated with agricultural plants. CABI, New York, pp 3–26
Larsson S, Ekbom B (1995) Oviposition mistakes in herbivorous insects: confusion or a step towards a new host giant. Oikos 72:155–160
Leather SR (1985) Oviposition preferences in relation to larval growth rates and survival in the pine beauty moth, Panolis flammea. Ecol Entomol 10:213–217
Lloyd DG (1987) Selection of offspring size at independence and other size-versus-number strategies. Am Nat 129:800–817
Mayhew PJ (1997) Adaptive patterns of host-plant selection by phytophagous insects. Oikos 79:417–428
Messina FJ (1982) Food plant choices of two goldenrod beetles: relation to plant quality. Oecologia 55:342–354
Mills NJ, Kuhlmann U (2000) The relationship between egg load and fecundity among Trichogramma parasitoids. Ecol Entomol 25:315–324
Nufio CR, Papaj DR (2004) Host marking behavior as a quantitative signal of infestation levels in host use by the walnut fly, Rhagoletis juglandis. Ecol Entomol 29:336–344
Nufio CR, Papaj DR, Alonso-Pimentel H (2000) Host utilization by the walnut fly, Rhagoletis juglandis (Diptera: Tephritidae). Environ Entomol 29:994–1001
Nylin S, Janz N (1996) Host plant preferences in the comma butterfly (Polygonia calbum): do parents and offspring agree? Ecoscience 3:285–289
Nylin S, Janz N, Wedell N (1996) Oviposition plant preference and offspring performance in the comma butterfly: correlations and conflicts. Entomol Exp Appl 80:141–144
Papaj DR (1993) Use and avoidance of occupied hosts as a dynamic process in tephritid fruit flies. In: Bernays EA (ed) Insect-Plant Interactions. CRC, Boca Raton, Fla., pp 25–46
Papaj DR (1994) Oviposition site guarding by male walnut flies and its possible consequences for mating success. Behav Ecol Sociobiol 34:187–195
Papaj DR, Alonso-Pimentel H (1997) Why walnut flies superparasitize: time savings as a possible explanation. Oecologia 109:166–174
Price PW, Ohgushi T (1995) Preference and performance linkage in a Phyllocolpa sawfly on the willow, Salix miyabeana, on hokkaido. Res Popul Ecol 37:23–28
Prokopy RJ (1981) Oviposition-deterring pheromone system of apple maggot flies. In: Mitchell EK (ed) Management of insect pests with semiochemicals. Plenum, New York, pp 477–494
Rausher MD (1980) Host abundance, juvenile survival, and oviposition preference in Battus philenor. Evolution 34:342–355
Rossi AM, Strong DR (1991) Effects of host-plant nitrogen on the preference and performance of laboratory populations of Carneocephala floridana (Homoptera: Cicadellidae). Environ Entomol 20:1349–1355
SAS (2000) JMP, Version 4. Statistics and Graphics Guide. SAS Institute, Cary, N.C.
Scheirs J, De Bruyn L (2002) Integrating optimal foraging and optimal oviposition theory in plant-insect research. Oikos 96:187–191
Scheirs J, De Bruyn L, Verhagen R (2000) Optimization of adult performance determines host choice in a grass miner. Proc R Soc Lond B Biol Sci 267:2065–2069
Smith RH, Lessells CM (1985) Oviposition, ovicide, and larval competition in granivorous insects. In: Sibly RM, Smith RH (eds) Behavioral ecology: ecological consequences of adaptive behaviour. Blackwell, Oxford, pp 423–448
Speirs DC, Sherratt TN, Hubbard SF (1991) Parasitoid diets—does superparasitism pay? Trends Ecol Evol 6:22–25
Taylor PW, Yuval B (1999) Postcopulatory sexual selection in Mediterranean fruit flies: advantages for large and protein fed males. Anim Behav 58:247–254
Thompson JN (1988) Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol Exp Appl 47:3–14
Valladares G, Lawton JH (1991) Host-plant selection in the holly leaf-miner: does mother know best? J Anim Ecol 60:227–240
Weisser WW, Houston AI, Volkl W (1994) Foraging strategies in solitary parasitoids: the trade-off between female and offspring mortality risks. Evol Ecol 8:587–597
Wickman PO, Karlsson B (1989) Abdomen size, body size and reproductive effort of insects. Oikos 56:209–214
Wiklund C (1981) Generalist vs. specialist oviposition behavior in Papilio machaon (Lepidoptera) and functional-aspects on the hierarchy of oviposition preferences. Oikos 36:163–170
Williams KS (1983) The coevolution of Euphydryas chalcedona butterflies and their larval host plants. III. Oviposition behavior and host plant quality. Oecologia 56:336–340
Acknowledgements
We thank Henar Alonso-Pimentel, Judie Bronstein, Reginald Chapman, Laurie Henneman, Dena Smith and the Plant-Animal Interactions reading group at the University of Colorado for comments and discussion. Sheridan Stone of the Fort Huachuca Wildlife Management Office of the United States of America Army provided permission and logistical support for fieldwork in Garden Canyon. A Pre-Graduate National Science Fellowship, NRICGP grant no. 93-37302-9126 to D. R. P., and Sigma Xi grant supported this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nufio, C.R., Papaj, D.R. Superparasitism of larval hosts by the walnut fly, Rhagoletis juglandis, and its implications for female and offspring performance. Oecologia 141, 460–467 (2004). https://doi.org/10.1007/s00442-004-1669-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1669-1