Abstract
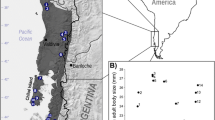
Bergmann’s rule states that, among conspecific populations, individuals are larger in cooler than in warmer environments as a consequence of selection related to heat conservation. Many of the most comprehensive assessments of Bergmann’s rule to date have examined clinal patterns in body size among species assemblages. Our study is a more direct test of Bergmann’s rule because we examine the pattern within a single, widely distributed species. We examined geographic variation in body and cell size in the spotted turtle (Clemmys guttata). Our analysis of 818 turtles collected from the entire range (45–28°N), indicated that body size increased with latitude; however, the relationship was driven by a population of large turtles at the northern extreme of the species’ range. When the northern population was removed from the analyses, Bergmann’s rule was not supported, and the smallest turtles occurred near the central part of the species’ distribution. Recent literature has suggested that latitudinal clines in body size may simply be a physiological byproduct of the effects of temperature on cell division, resulting in larger cells, and hence larger organisms, from cooler temperatures. Measurements of the diameter of skin cells did not support the hypothesis that cell size increases with latitude and decreases with temperature in the spotted turtle, nor was there a significant relationship between body size and cell size. Our study suggests that neither Bergmann’s rule nor cell size variation sufficiently explain the body size cline observed in the spotted turtle. We hypothesize that patterns in body size are related to variation in female size at maturity and reproductive cycles.





Similar content being viewed by others
References
Arnett AE, Gotelli NJ (1999) Geographic variation in life-history traits of the ant lion, Myrmeleon immaculatus: evolutionary implications of Bergmann’s rule. Evolution 53:1180–1188
Ashton KG (2001) Are ecological and evolutionary rules being dismissed prematurely? Divers Distributions 7:289–295
Ashton KG (2002a) Patterns of within-species body size variation of birds: strong evidence for Bergmann’s rule. Global Ecol Biogeogr 11:505–523
Ashton KG (2002b) Do amphibians follow Bergmann’s rule? Can J Zool 80:708–716
Ashton KG, Feldman CR (2003) Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57:1151–1163
Ashton KG, Tracy MC, de Queiroz A (2000) Is Bergmann’s rule valid for mammals? Am Nat 156:390–415
Atkinson D (1994) Temperature and organism size—a biological law for ectotherms? Adv Ecol Res 25:1–58
Atkinson D, Sibly RM (1997) Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol Evol 12:235–239
Belk MC, Houston DD (2002) Bergmann’s rule in ectotherms: a test using freshwater fishes. Am Nat 160:803–808
Bergmann C (1847) Ueber die verhaeltnisse der waermeoekonomie der thiere zu ihrer groesse. Goettinger Studien 1:595–708
Berven KA, Gill DE (1983) Interpreting geographic variation in life-history traits. Am Zool 23:85–97
Blanckenhorn WU, Fairbairn DJ (1995) Life history adaptation along a latitudinal cline in water striders, Aquarius remigis. J Evol Biol 8:21–41
Blanckenhorn WU, Hellriegel B (2002) Against Bergmann’s rule: fly sperm size increases with temperature. Ecol Lett 5:7–10
Boyce MS (1978) Climatic variability and body size variation in the muskrats (Ondatra zibethicus) of North America. Oecologia 36:1–20
Brooks RJ, Shilton CM, Brown GP, Quinn NWS (1992) Body size, distribution, and reproduction in a northern population of wood turtles (Clemmys insculpta). Can J Zool 70:462–469
Calder WA III (1974) Consequences of body size for avian energetics. In: Paynter RA Jr (ed) Avian energetics. Nuttall Ornithology Club, Cambridge, pp 86–151
Edmonds JH, Brooks RJ (1996) Demography, sex ratio, and sexual size dimorphism in a northern population of stinkpot turtles (Sternotherus odoratus). Can J Zool 74:918–925
Ernst CH (1970) Reproduction in Clemmys guttata. Herpetologica 26:228–232
Ernst CH, Lovich JE, Barbour RW (1994) Turtles of the United States and Canada. Smithsonian Institution Press, Washington, D.C.
French V, Feast M, Partridge L (1998) Body size and cell size in Drosophila: the developmental response to temperature. J Insect Physiol 44:1081–1089
Galbraith DA, Brooks RJ, Obbard ME (1989) The influence of growth rate on age and body size at maturity in female snapping turtles (Chelydra serpentina). Copeia 1989:896–904
Gibbons JW, Lovich JE (1990) Sexual dimorphism in turtles with emphasis on the slider turtle (Trachemys scripta). Herpetol Monogr 4:1–29
Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L (2000) Rapid evolution of a geographic cline in size in an introduced fly. Science 287:308–309
James FC (1970) Geographic size variation in birds and its relationship to climate. Ecology 51:365–390
James AC, Azevedo RBR, Partridge L (1995) Cellular basis and developmental timing in a size cline of Drosophila melanogaster. Genetics 140:659–666
Johansson F (2003) Latitudinal shifts in body size of Enallagma cyathigerum (Odonata). J Biogeogr 30:29–34
Katti M, Price TD (2003) Latitudinal trends in body size among over-wintering leaf warblers (genus Phylloscopus). Ecography 26:69–79
Knouft JH (2002) Regional analysis of body size and population density in stream fish assemblages: testing predictions of the energetic equivalence rule. Can J Fish Aquat Sci 59:1350–1360
Lindsey CC (1966) Body sizes of poikilotherm vertebrates at different latitudes. Evolution 20:456–465
Litzgus JD, Brooks RJ (1998a) Growth in a cold environment: body size and sexual maturity in a northern population of spotted turtles, Clemmys guttata. Can J Zool 76:773–782
Litzgus JD, Brooks RJ (1998b) Reproduction in a northern population of Clemmys guttata. J Herpetol 32:252–259
Litzgus JD, Mousseau TA (2003) Multiple clutching in southern spotted turtles, Clemmys guttata. J Herpetol 37:17–23
Masaki S (1967) Geographic variation and climatic adaptation in a field cricket (Orthoptera: Gryllidae). Evolution 21:725–741
Mayr E (1956) Geographical character gradients and climatic adaptations. Evolution 10:105–108
Meiri S, Dayan T (2003) On the validity of Bergmann’s rule. J Biogeogr 30:331–351
Morrison P (1960) Some interrelations between weight and hibernation function. Bull Mus Comp Zool 124:75–91
Mousseau TA (1997) Ectotherms follow the converse to Bergmann’s rule. Evolution 51:630–632
Mousseau TA (2000) Intra- and interpopulation genetic variation, explaining the past and predicting the future. In: Mousseau TA, Sinervo B, Endler J (eds) Adaptive genetic variation in the wild. Oxford University Press, New York, pp 219–250
Mousseau TA, Roff DA (1989) Adaptation to seasonality in a cricket: patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution 43:1483–1496
Murphy EC (1985) Bergmann’s rule, seasonality, and geographic variation in body size of house sparrows. Evolution 39:1327–1334
Partridge L, Barrie B, Fowler K, French V (1994) Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48:1269–1276
Petraitis PS, Beaupre SJ, Dunham AE (2001) ANCOVA: nonparametric and randomization approaches. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, New York, pp 116–133
Ray C (1960) The application of Bergmann’s and Allen’s rules to the poikilotherms. J Morphol 106:85–108
Reed RN (2003) Interspecific patterns of species richness, geographic range size, and body size among New World venomous snakes. Ecography 26:107–117
Roff DA (1980) Optimizing development time in a seasonal environment: the “ups and downs” of clinal variation. Oecologia 45:202–208
Roff DA (1983) Phenological adaptation in a seasonal environment: a theoretical perspective. In: Brown VK, Hodek I (eds) Diapause and life cycle strategies in insects. Junk, The Hague, pp 253–270
Roff DA (1992) The Evolution of life histories: theory and analysis. Chapman and Hall, New York
Roff DA (1997) Evolutionary quantitative genetics. Chapman and Hall, New York
Schluter D (2000) Estimating fitness functions using the cubic spline. Department of Zoology, University of British Columbia, Vancouver
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Tinkle DW (1961) Geographic variation in reproduction, size, sex ratio and maturity of Sternotherus odoratus. Ecology 42:68–76
Van der Have TM, de Jong G (1996) Adult size in ectotherms: temperature effects on growth and differentiation. J Theor Biol 183:329–340
Van Voorhies WA (1996) Bergmann size clines: a simple explanation for their occurrence in ectotherms. Evolution 50:1259–1264
Wilbur HM, Morin PJ (1988) Life history evolution in turtles. In: Gans C, Huey RB (eds) Biology of the reptilia, vol. 16, ecology B-defense and life history. Alan R. Liss, New York, pp 387–437
Willemsen RE, Hailey A (1999) Variation of adult body size of the tortoise Testudo hermanni in Greece: proximate and ultimate causes. J Zool 248:379–396
Yom-Tov Y, Benjamini Y, Kark S (2002) Global warming, Bergmann’s rule and body mass are they related? The chukar partridge (Alectoris chukar) case. J Zool 257:449–455
Zink RM, Remsen JV Jr (1986) Evolutionary processes and patterns of geographic variation in birds. In: Johnston RF (ed) Current ornithology, vol 4. Plenum, New York, pp 1–69
Acknowledgements
This research was supported by grants from the National Geographic Society (Grant No. 6630-99), the Santee Cooper power company, the Chelonian Research Foundation, Sigma Xi, the Carnegie Museum of Natural History, the SC Wildlife Federation, a Howard Hughes Undergraduate Research Scholarship (to S.E.D.), and the National Science Foundation (grant no. 0090177 to T.A.M.). We thank the following museum curators who allowed us access to their collections: Craig Guyer (Auburn University Museum of Natural History), Max Nickerson (Florida Museum of Natural History, University of Florida), Alan Resetar (Chicago Field Museum of Natural History), Dave Rostal (Georgia Southern University, formerly the Savannah Science Museum), John Wiens (Carnegie Museum of Natural History), and George Zug (Smithsonian Institution National Museum of Natural History). W.J. Humphries provided body size data from live turtles at his study site in West Virginia, and R. Walters and R. Leonard allowed us access to their collections of live turtles captured in southern South Carolina. In-kind support was provided by the Francis Beidler Forest National Audubon and Nature Conservancy Sanctuary (Harleyville, S.C.), and J.D. Congdon (SREL, Aiken, S.C.). J. Bixby, A. Stallings, N. Heslop, W.J. Humphries, and T.J.S. Merritt assisted with data collection. K. Hural and T.J.S. Merritt reviewed an earlier draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Litzgus, J.D., DuRant, S.E. & Mousseau, T.A. Clinal variation in body and cell size in a widely distributed vertebrate ectotherm. Oecologia 140, 551–558 (2004). https://doi.org/10.1007/s00442-004-1611-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1611-6