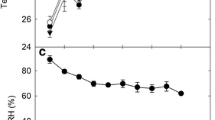
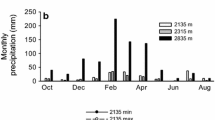
Abstract
Water status in relation to standing biomass and leaf area indices (LAI) of the subtropical foredune species Arctotheca populifolia, Ipomoea pes-caprae and Scaevola plumieri were studied in the Eastern Cape, South Africa. The plants showed little evidence of water stress, never developing leaf water potentials more negative than −1.55 MPa, a value which is typical of mesophytes rather than xerophytes. The plants showed no seasonal changes in osmotic potential, an indication that they did not need to osmoregulate, nor were there significant alterations in tissue elasticity. Turgor potential for the most part remained positive throughout the day or recovered positive values at night, a condition suitable for the maintenance of growth that may be essential to cope with sand accretion. All three species show relatively high transpiration rates and only I. pes-caprae showed any evidence of strong limitations of transpiration rate through reductions in midday stomatal conductance. All three species had relatively high instantaneous water use efficiencies as a result of high assimilation rates rather than low transpiration rates. Simple water budgets, accounting for losses by transpiration and inputs from rainfall, suggest that the water stored in the dune sands is sufficient to meet the requirements of the plants, although water budgets calculated for I. pes-caprae suggest that this species may on occasion be water limited. The results suggest that it is the low biomass and LAI that lead to these favourable water relations.


Similar content being viewed by others
References
Bach CE (1998) Interactive effects of herbivory and sand burial on growth of a tropical dune plant, Ipomoea pes-caprae. Ecol Entomol 23:238–245
Barbour MG (1990) The coastal beach plant syndrome. Proc Can Symp Coastal Sand Dunes 1990:197–214
Barbour MG, DeJong TM, Pavlik BM (1985) Marine beach and dune plant communities. In: Chabot BF, Mooney HA (eds) Physiological ecology of North American plant communities. Chapman and Hall, New York, pp 296–322
Boyer JS (1995) Measuring the water status of plants and soils. Academic Press, USA
Caldwell D (1985) Cold desert. In: Chabot BF, Mooney HA (eds) Physiological ecology of North American plant communities. Chapman and Hall, London, pp 198–212
Caldwell MM, White RW, Moore RT, Camp LB (1977) Carbon balance, productivity and water use of cold-winter desert shrub communities dominated by C3 and C4 species. Oecologia 29:275–300
Davy AJ, Figueroa ME (1993) The colonization of strandlines. In: Miles J, Walton DWH (eds) Primary succession on land. (Special publication series of the British Ecological Society, no. 12). Blackwell, Oxford, pp 113–131
De Jong TM (1978) Comparative gas exchange of four California beach taxa. Oecologia 34:343–351
De Jong TM (1979) Water and salinity relations of Californian beach species. J Ecol 67:647–663
Garcia-Novo F (1979) The ecology of vegetation of the dunes in Donana National Park (south-west Spain). In: Jefferies RL, Davy AJ (eds) Ecological processes in coastal environments. Blackwell, Oxford, pp 571–592
Goff JA, Gatch S (1946) Low-pressure properties of water from −160°F to 212°F. Trans Am Soc Heat Ventil Eng 52:95–121
Grootjans AP, Ernst WHO, Stuyfzand PJ (1998) European dune slacks: strong interactions of biology, pedogenesis and hydrology. Trees 13:96–100
Hanson AD, Hitz WD (1982) Metabolic responses of mesophytes to plant water deficits. Annu Rev Plant Physiol 33:163–203
Harte JM, Pammenter NW (1983) Leaf nutrient content in relation to senescence in the coastal sand dune pioneer Scaevola thunbergii Eckl. & Zeyh. S Afr J Sci 79:420–422
Hesp PA (1991) Ecological processes and plant adaptations on coastal dunes. J Arid Environ 21:165–191
Huiskes AHL (1979) Ammophila arenaria (L.) Link. biological flora of the British Isles. J Ecol 67:363–382
Jefferies RL, Davy AJ, Rudmik T (1977) The growth strategies of coastal halophytes. In: Jefferies RL, Davy AJ (eds), Ecological processes in coastal environments. Blackwell, London, pp 243–268
Lambers H, Chapin FS, Pons TL (1998) Plant physiological ecology. Springer, Berlin Heidelberg New York
Larcher W (2003) Physiological plant ecology. Ecophysiology and stress physiology of functional groups, 4th edn. Springer, Berlin Heidelberg New York
Martinez ML, Moreno-Casasola P (1998) The biological flora of coastal dunes and wetlands: Chamaecrista chamaecristoides (Colladon) I. J Coastal Res 14(1):162–174
Miller PC, Miller JM, Miller PM (1983) Seasonal progression of plant water relations in fynbos in the Western Cape Province, South Africa. Oecologia 56:392–396
Mooney HA, Kummerow J, Johnson AW, Parsons DJ, Keeley S, Hoffmann A, Hays RI, Gilberto J, Chu C (1977) The producers—their resources and adaptive responses. In: Mooney HA (ed) Convergent evolution in Chile and California. Dowden, Hutchinson and Ross, Stroudsburg, Pa., pp 85–143
Olsson-Seffer P (1909) Hydrodynamic factors influencing plant life on sandy seashores. New Phytol 8:37–49
Oosting HJ (1954) Ecological processes of vegetation of maritime strand in the south eastern United States. Bot Rev 20:226–262
Pammenter NW (1983) Some aspects of the ecophysiology of Scaevola thunbergii, a subtropical dune pioneer. In: Swart DH, Eagle GA, McLachlan A, Brown AC, Heydorn AEF, Herbst F (eds) Sandy beaches as ecosystems. Junk, the Hague, pp 675–698
Pammenter NW (1985) Photosynthesis and transpiration of the subtropical coastal sand dune pioneer Scaevola plumieri under controlled conditions. S Afr J Bot 51(6):421–424
Pavlik BM (1983) Nutrient and productivity relations of the dune grass Ammophila arenaria and Elymus mollis. I. Blade photosynthesis and nitrogen use efficiency in the laboratory and field. Oecologia 57:233–238
Pavlik BM (1984) Seasonal changes of osmotic pressure, symplastic water content and tissue elasticity in the blades of dune grasses growing in situ along the coast of Oregon. Plant Cell Environ 7:531–539
Pavlik BM (1985) Water relations of the dune grasses Ammophila arenaria and Elymus mollis on the coast of Oregon, USA. Oikos 45:197–205
Peter CI, Ripley BS (2000) An empirical formula for estimating the water use of Scaevola plumieri. S Afr J Sci 96:593–596
Ranwell DS (1972) Ecology of salt marshes and sand dunes. Chapman and Hall, London
Richter H (1997) Water relations of plants in the field: some comments on the measurement of selected parameters. J Exp Bot 48, 306, 1–7
Ripley BS, Pammenter NW (2004) Physiological characteristics of coastal dune pioneer species from the Eastern Cape, South Africa, in relation to stress and disturbance. In: Martínez M, Psuty NP (eds) Ecological Studies, vol 171. Coastal dunes: ecology and conservation. Springer, Berlin Heidelberg New York, pp 137–154
Ripley BS, Pammenter NW, Smith VR (1999) Function of leaf hairs revisited: the hair layer on leaves of Arctotheca populifolia reduces photoinhibition, but leads to higher leaf temperatures caused by lower transpiration rates. J Plant Physiol 155:78–85
Rozema JP, Bijwaard G, Prast G, Broekman R (1985) Ecophysiological adaptations of coastal halophytes from foredunes and salt marshes. Vegetatio 62:499–521
Salisbury E (1952) Downs and dunes. Their plant life and its environment. Bell, London
Scholes RJ, Walker BH (1993) An African savanna: synthesis of the Nylsvley study. Cambridge University Press, Cambridge
Schulze E-D, Hall AE (1982) Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology, new series12B. Springer, Berlin Heidelberg New York, pp 181–230
Schulze E-D, Lange OL, Kappen L, Buschbom U (1974) The role of air humidity and leaf temperature in controlling stomatal resistance of Prunus armeniaca L. under desert conditions. I. A simulation of the daily course of stomatal resistance. Oecologia 17:159–170
Schulze E-D, Lange OL, Kappen L, Evenari M, Buschbom U (1975) The role of air humidity and leaf temperature in controlling stomatal resistance of Prunus armeniaca L. under desert conditions. II. The significance of leaf water status and internal carbon dioxide concentration. Oecologia 18:219–233
Schulze E-D, Lange OL, Kappen L, Evenari M, Buschbom U (1980) Long-term effects of drought on wild and cultivated plants in the Negev desert. I. Maximal rates of photosynthesis. Oecologia 45:11–18
Sinclair R, Venables WN (1983) An alternative method for analysing pressure-volume curves produced with the pressure chamber. Plant Cell Environ 6:211–217
Smith WK (1985) Western montane forests. In: Chabot BF, Mooney HA (eds) Physiological ecology of North American plant communities. Chapman and Hall, London, pp 95–126
Tinley KL (1985) Coastal dunes of South Africa. South African National Scientific Programmes report no. 109. Foundation for Research Development, Council for Scientific and Industrial Research, Pretoria
Tyree MT (1976) Negative turgor pressure in plant cells: fact or fallacy. Can J Bot 54:2738–2746
Tyree MJ, Jarvis PG (1982) Water in tissues and cells In: Lange OL, Nobel PS, Osmond CB, Zeigler H (eds) Encyclopedia of plant physiology, new series 12B. Springer, Berlin Heidelberg New York, pp 35–77
Valk AG van der (1974) The environmental factors controlling foredune plant communities in Cape Hatteras National Seashore. Ecology 55:1349–1358
Willert DJ von, Herppich M, Miller JM (1989) Photosynthetic characteristics and leaf water relations of mountain fynbos vegetation in the Cedarberg area (South Africa). S Afr J Bot 55(3):288–298
Willis AJ, Jefferies RL (1963) Investigations on the plant water relations of sand-dune plants under natural conditions. In: Rutter AJ, Whitehead FH (eds) The water relations of plants. Blackwell, Oxford, pp 168–189
Wilson DE (1977) Ecological observations on the tropical strand plants Ipomoea pes-caprae (L.) R. Br. (Convolvulaceae) and Canavalia maritima (Aubl.) Thou. (Fabaceae). Brenesia 31–42
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ripley, B.S., Pammenter, N.W. Do low standing biomass and leaf area index of sub-tropical coastal dunes ensure that plants have an adequate supply of water?. Oecologia 139, 535–544 (2004). https://doi.org/10.1007/s00442-004-1535-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1535-1