Abstract
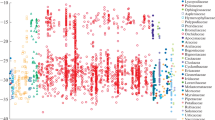
The occurrence of crassulacean acid metabolism (CAM) in the epiphyte community of a lowland forest of the Atlantic slope of Panama was investigated. I hypothesized that CAM is mostly found in orchids, of which many species are relatively small and/or rare. Thus, the relative proportion of species with CAM should not be a good indicator for the prevalence of this photosynthetic pathway in a community when expressed on an individual or a biomass basis. In 0.4 ha of forest, 103 species of vascular epiphytes with 13,099 individuals were found. As judged from the C isotope ratios and the absence of Kranz anatomy, CAM was detected in 20 species (19.4% of the total), which were members of the families Orchidaceae, Bromeliaceae, and Cactaceae. As predicted, the contribution of CAM epiphytes to the total number of individuals and to total biomass (69.6 kg ha-1) was considerably lower (3.6% or 466 individuals and, respectively, 3.0% or 2.1 kg ha-1).




Similar content being viewed by others
References
Coutinho LM (1969) Novas observações sôbre a ocorrência do “efeito de de Saussure” e suas relações com a suculência, a temperatura folhear e os movimentos estomáticos. Botanica 24:77–102
Croat TB (1978) Flora of Barro Colorado Island. Stanford University Press, Stanford
D’Arcy WG (1987) Flora of Panama: checklist and index. Missouri Botanical Garden, St. Louis, Mo.
Dressler RL (1981) The orchids. Natural history and classification. Harvard University Press, Cambridge, Mass.
Dressler RL (1993) Field guide to the orchids of Costa Rica and Panama. Cornell University Press, Ithaka, N.Y.
Earnshaw MJ, Winter K, Ziegler H, Stichler W, Cruttwell NEG, Kerenga K, Cribb PJ, Wood J, Croft JR, Carver KA, Gunn TC (1987) Altitudinal changes in the incidence of crassulacean acid metabolism in vascular epiphytes and related life forms in Papua New Guinea. Oecologia 73:566–572
Edwards PJ, Grubb PJ (1977) Studies of mineral cycling in a montane rainforest in New Guinea. I. The distribution of organic matter in the vegetation and soil. J Ecol 65:943–969
Evenari M (1985) Adapations of plants and animals to the desert environment. In: Evenari M, Noy-Meir I, Goodall DW (eds.) Hot deserts and arid shrublands. A. Ecosystems of the world vol 12A. Elsevier, Amsterdam, pp 79–92
Flores-Palacios A, Garcia FJG (2001) Sampling methods for vascular epiphytes: their effectiveness in recording species richness and frequency. Selbyana 22:181–191
Frangi JL, Lugo AE (1992) Ecosystem dynamics of a subtropical floodplain forest. Ecol Monogr 55:351–369
Gentry AH, Dodson CH (1987) Diversity and biogeography of neotropical vascular epiphytes. Ann Mo Bot Gard 74:205–233
Golley FB, McGinnis JT, Clements RG, Child GI, Duever MJ (1969) The structure of tropical forests in Panama and Colombia. BioScience 19:693–696
Griffiths H, Smith JAC (1983) Photosynthetic pathways in the Bromeliaceae of Trinidad: relations between life-forms, habitat preference and the occurrence of CAM. Oecologia 60:176–184
Griffiths H, Lüttge U, Stimmel KH, Crook CE, Griffiths NM, Smith JAC (1986) Comparative ecophysiology of CAM and C3 bromeliads. III. Environmental influences on CO2 assimilation and transpiration. Plant Cell Environ 9:385–393
Hietz P, Hietz-Seifert U (1995) Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, Mexico. J Veg Sci 6:487–498
Hietz P, Wanek W, Popp M (1999) Stable isotopic composition of carbon and nitrogen, and nitrogen content in vascular epiphytes along an altitudinal transect. Plant Cell Environ 22:1435–1443
Hsu C-C, Horng F-W, Kuo C-M (2002) Epiphyte biomass and nutrient capital of a moist subtropical forest in north-eastern Taiwan. J Trop Ecol 18:659–670
Klinge H, Rodriguez WA, Bruning E, Fittkau EJ (1975) Biomass and structure in a Central Amazonian rain forest. In: Golley FB, Medina E (eds) Tropical ecological systems. (Ecological studies vol 11) Springer, Berlin Heidelberg New York, pp 115–122
Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. Part I. Psilotaceae through Dicksoniaceae. American Fern Society, Washington, D.C.
Lerdau MT, Throop HL (1999) Isoprene emission and photosynthesis in a tropical forest canopy: implications for model development. Ecol Appl 9:1109–1117
Liang YM, Hazlett DL, Lauenroth WK (1989) Biomass dynamics and water use efficiencies of five plant communities in the shortgrass steppe. Oecologia 80:148–153
Lüttge U (1997) Physiological ecology of tropical plants. Springer, Berlin Heidelberg New York
Mooney HA, Bullock SH, Ehleringer JR (1989) Carbon isotope ratios of plants of a tropical forest in Mexico. Funct Ecol 3:137–142
Murphy PG, Lugo AE (1986) Structure and biomass of a subtropical dry forest in Puerto Rico. Biotropica 18:89–96
Nadkarni NM (1984) Epiphyte biomass and nutrient capital of a neotropical elfin forest. Biotropica 16:249–256
Nieder J, Engwald S, Klawun M, Barthlott W (2000) Spatial distribution of vascular epiphytes (including hemiepiphytes) in a lowland Amazonian rain forest (Surumoni Crane Plot) of Southern Venezuela. Biotropica 32:385–396
Nuernbergk EL (1960) Endogener Rhythmus und CO2-Stoffwechsel bei Pflanzen mit diurnalem Säurerhythmus. Planta 56:28–70
Osmond CB, Winter K, Ziegler H (1982) Functional significance of different pathways of CO2 fixation in photosynthesis. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology. II. Encylopedia of Plant Physiology, new series, vol 12B. Springer, Berlin Heidelberg New York, pp 479–548
Pierce S, Winter K, Griffiths H (2002a) Carbon isotope ratio and the extent of daily CAM use by Bromeliaceae. New Phytol 156:75–83
Pierce S, Winter K, Griffiths H (2002b) The role of CAM in high rainfall cloud forests: an in situ comparison of photosynthetic pathways in Bromeliaceae. Plant Cell Environ 25:1181–1189
Rundel PW, Palma B, Dillon MO, Sharifi MR, Nilsen ET, Boonpragob K (1997) Tillandsia landbeckii in the coastal Atacama Desert of northern Chile. Rev Chil Hist Nat 70:341–349
Sanford WW (1968) Distribution of epiphytic orchids in semi-deciduous tropical forest in southern Nigeria. J Ecol 56:697–705
Smith JAC, Winter K (1996) Taxonomic distribution of crassulacean acid metabolism. In: Winter K, Smith JAC (eds) Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. (Ecological studies) Springer, Berlin Heidelberg New York, pp 427–436
Smith SD, Monson RK, Anderson JE (1997) Physiological ecology of North American desert plants. Springer, Berlin Heidelberg New York
Tanner EVJ (1980) Studies on the biomass and productivity in a series of montane rain forests in Jamaica. J Ecol 68:573–588
Tremblay RL, Zimmerman JK, Lebrón L, Bayman P, Sastre I, Axelrod F, Alers-García J (1998) Host specifity and low reproductive success in the rare endemic Puerto Rican orchid Lepanthes caritensis. Biol Conserv 85:297–304
Troughton JH (1979) δ13C as an indicator of carboxylation reactions. In: Gibbs M, Latzko E (eds) Photosynthesis. II. Photosynthetic carbon metabolism. Encylopaedia of plant physiology, new series, vol 6. Springer, Berlin Heidelberg New York, pp 140–147
Wanek W, Huber W, Arndt SK, Popp M (2002) Mode of photosynthesis during different life stages of hemiepiphytic Clusia species. Funct Plant Biol 29:725–732
Winter K (1985) Crassulacean acid metabolism. In: Barber J, Baker NR (eds) Photosynthetic mechanisms and the environment. Topics in photosynthesis vol 6. Elsevier, Amsterdam, pp 329–387
Winter K, Holtum JAM (2002) How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiol 129:1843–1851
Winter K, Smith JAC (1996) An introduction to crassulacean acid metabolism: biochemical principles and biological diversity. In: Winter K, Smith JAC (eds) Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. (Ecological studies) Springer, Berlin Heidelberg New York, pp 1–13
Winter K, Wallace BJ, Stocker GC, Roksandic Z (1983) Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia 57:129–141
Zotz G, Büche M (2000) The epiphytic filmy ferns of a tropical lowland forest—species occurrence and habitat preferences. Ecotropica 6:203–206
Zotz G, Vollrath B (2003) The epiphyte vegetation of the palm, Socratea exorrhiza —correlations with tree size, tree age, and bryophyte cover. J Trop Ecol 19:81–90
Zotz G, Ziegler H (1997) The occurrence of crassulacean acid metabolism among vascular epiphytes from Central Panama. New Phytol 137:223–229
Zotz G, Ziegler H (1999) Size-related differences in carbon isotope discrimination in the epiphytic orchid, Dimerandra emarginata. Naturwissenschaften 86:39–40
Zotz G, Bermejo P, Dietz H (1999) The epiphyte vegetation of Annona glabra on Barro Colorado Island, Panama. J Biogeogr 26:761–776
Acknowledgements
The excellent assistance of S. Schultz (Würzburg, Germany) was essential for the success of this study. Funds came from the Smithsonian Tropical Research Institute (STRI) (Panama), the United Nations Environmental Program, the Deutsche Forschungsgemeinschaft, the FAG, Basel, Switzerland, and the A. F. W. Schimperstiftung, Stuttgart, Germany. Thanks to S. J. Wright, V. Horlyck, J. Herrera and E. Andrade (all STRI) for organising work at the Crane. Help with the identification of epiphytes from D. Lellinger (Washington, D.C.), J. Atwood (Sarasota, USA), R. Dressler (Florida), and C. Galdames (STRI) is appreciated. P. Hietz (Vienna) did not hesitate to dig out decade-old data. Finally, many thanks to W. Wanek (Vienna) for help with the mass spectrometry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zotz, G. How prevalent is crassulacean acid metabolism among vascular epiphytes?. Oecologia 138, 184–192 (2004). https://doi.org/10.1007/s00442-003-1418-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1418-x