Abstract
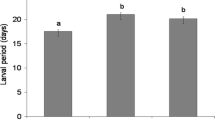
Larvae of the leaf beetle Chrysomela lapponica obtain salicyl glucosides (SGs) from the host plant to produce a defensive secretion with salicylaldehyde. In northern Russia, larvae and pupae experience high parasitism by the phorid fly Megaselia opacicornis and tachinid fly Cleonice nitidiuscula. We compared the suitability of the SG-rich Salix borealis and SG-poor S. caprea and S. phylicifolia to Ch. lapponica and tested whether enemy pressure on Ch. lapponica varies among host species that differ in SG content. In the laboratory, survival of Ch. lapponica larvae was higher on S. borealis than on S. caprea and S. phylicifolia, while adult body mass was higher on S. borealis and S. caprea than on S. phylicifolia. In the field, parasitism by both M. opacicornis and Cl. nitidiuscula was greater on beetles from S. borealis than from the SG-poor S. caprea or S. phylicifolia. In a laboratory choice test, the pupal parasitoid M. opacicornis laid similar numbers of eggs on beetles reared on SG-rich and SG-poor willows, suggesting that the host plant-derived defence is not effective against this parasitoid. In a field enemy-exclusion experiment, beetle survival was greatly enhanced by the exclusion of enemies, but survival rates did not differ between S. borealis and S. caprea, although larvae developed faster on S. borealis. On the other hand, parasitism and predation were observed more often on S. borealis than on S. caprea. Thus, beetle larvae perform better but also suffer higher predation and parasitism on S. borealis than on SG-poor willows. Ch. lapponica does not appear to obtain enemy-free space by feeding on SG-rich willow species.




Similar content being viewed by others
References
Ballabeni P, Wlodarczyk M, Rahier M (2001) Does enemy-free space for eggs contribute to a leaf beetle's oviposition preference for nutritionally inferior host plant? Funct Ecol 15:318–324
Baur R, Rank NE (1996) Influence of host quality and natural enemies on the life history of the alder leaf beetles Agelastica alni and Linaeidea aenea. In: Jolivet PH, Cox ML (eds) Chrysomelidae biology, vol 2. Ecological studies. SPB, Amsterdam, pp 173–194
Benrey B, Callejas A, Rios L, Oyama K, Denno RF (1998) The effects of domestication of Brassica and Phaseolus on the interaction between phytophagous insects and parasitoids. Biol Contr 11:130–140
Berdegue M, Trumble JT, Hare JD, Redak RA (1996) Is it enemy-free space? The evidence for terrestrial insects and freshwater arthropods. Ecol Entomol 21:203–217.
Bernays EA (1988) Host specificity in phytophagous insects: selection pressure from generalist predators. Entomol Exp Appl 49:131–140
Bernays E, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Blumberg D (1991) Seasonal variations in the encapsulation of eggs of the encyrtid parasitoid Metaphycus stanleyi by the pyriform scale, Protopulvinaria pyriformis. Entomol Exp Appl 58:231–237
Blumberg D (1997) Parasitoid encapsulation as a defence mechanism in the coccoidea (Homoptera) and its importance in biological control. Biol Contr 8:225–236
Bourchier RS (1991) Growth and development of Compsilura concinnata (Meigan) (Diptera: Tachinidae) parasitizing gypsy moth larvae feeding on tannin diets. Can Entomol 123:1047–1056
Burkot TR, Benjamin DM (1979) The biology and ecology of the cottonwood leaf beetle, Chrysomela scripta (Coleoptera: Chrysomelidae), on tissue cultured hybrid Aigeiros (Populus × euramericana) subclones in Wisconsin. Can Entomol 111:551–556
Campbell CAM, Duffy SS (1979) Tomatine and parasitic wasps: potential incompatibility of plant antibiosis with biological control. Science 205:700–702
Charnov EL, Skinner SW (1984) Evolution of host selection and clutch size in parasitoid wasps. Fla Entomol 67:5–21
Cox ML (1994) The Hymenoptera and Diptera parasitoids of Chrysomelidae. In: Jolivet PH, Cox ML, Petitpierre E (eds) Novel aspects of the biology of Chrysomelidae. Kluwer, Dordrecht, pp 419–467
Denno RF, Larsson S, Olmstead KL (1990) Role of enemy-free space and plant quality in host-plant selection by willow beetles. Ecology 71:124–137
Devantoy J (1948) Les Prédateurs et les parasites de la chrysomèle du peuplier. Feuille Nat 3:85–89
Dicke M, van Poecke MP (2002) Signalling in plant–insect interactions: signal transduction in direct and indirect plant defence. In: Scheel D, Wasternack C (eds) Plant signal transduction. Oxford University Press, New York, pp 289–316
Disney RHL, Zvereva EL, Mostovski MB (2001) A scuttle fly (Diptera: Phoridae) parasitizing a beetle (Coleoptera: Chrysomelidae) in Russia. Entomol Fenn 12:59–63
English-Loeb GM, Brody AK, Karban R (1993) Host-plant-mediated interactions between a generalist foliavore and its tachinid parasitoid. J Anim Ecol 62:465–471
Feder JL (1995) The effects of parasitoids on sympatric host races of Rhagoletis pomonella (Diptera: Tephritidae). Ecology 76:801–813
Godfray HCJ (1994) Parasitoids. Behavioural and evolutionary ecology. Princeton University Press, Princeton, N.J.
Gratton C, Welter SC (1999) Does "enemy-free space" exist? Experimental host shifts of an herbivorous fly. Ecology 80:773–785
Gross J (2001) On the evolution of host plant specialization in leaf beetles (Coleoptera: Chrysomelina). Inaugural-dissertation zur Erlangung des Doktorgrades. Institut für Biology — Angewandte Zoologie/Ökologie der Tiere, Freien Universität Berlin, Logos, Berlin
Gross J, Hilker M (1995) Chemoecological studies of the exocrine glandular larval secretion of two chrysomelid species (Coleoptera): Phaedon cochleariae and Chrysomela lapponica. Chemoecology 5/6:185–189
Gruenhagen NM, Perring TM (2001) Plant influences on silverleaf whitefly oviposition and development and the potential for enemy-free space. Entomol Exp Appl 99:387–391
Hilker M, Schulz S (1994) Composition of larval secretion of Chrysomela lapponica (Coleoptera, Chrysomelidae) and its dependence on host plant. J Chem Ecol 20:1075–1093
Horton DR (1989) Performance of a willow-feeding beetle, Chrysomela knabi Brown, as affected by host species and dietary moisture. Can Entomol 121:777–780
Jeffries MJ, Lawton J (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Jervis MA, Copland MJV (1996) The life cycle. In: Jervis M, Kidd N (eds) Insect natural enemies: practical approaches to their study and evaluation. Chapman and Hall, London, pp 63–161
Julkunen-Tiitto R (1989a) Distribution of certain phenolics in Salix species (Salicaceae). Univ Joensuu Publ Sci 15:1–19
Julkunen-Tiitto R (1989b) Phenolic constituents of Salix: a chemotaxonomic survey of further Finnish species. Phytochemistry 28:2115–2125
Kanervo V (1939) Beobachtungen und Versuche zur Ermittlung der Nahrung einiger Coccinelliden (Col.). Ann Entomol Fenn 5:89–110
Kanervo V (1946) Tutkimuksia lepän lehtikuoriaisen, Melasoma aenea L. (Col., Chrysomelidae), luontaisista vihollisista.. Ann Zool Soc Zool Bot Fenn Vanamo 12:1–202
Keese MC (1997) Does escape to enemy-free space explain host specialization in two closely related leaf-feeding beetles (Coleoptera: Chrysomelidae)? Oecologia 112:81–86
Kolehmainen J, Julkunen-Tiitto R, Roininen H, Tahvanainen J (1995) Phenolic glucosides as feeding cues for willow–feeding leaf beetles. Entomol Exp Appl 74:235–243
Köpf A, Rank N, Roininen H, Tahvanainen J (1997) Defensive larval secretions of leaf beetles attract a specialist predator Parasyrphus nigritarsis. Ecol Entomol 22:176–183
Köpf A, Rank NE, Roininen H, Julkunen-Tiitto R, Pasteels JM, Tahvanainen J (1998) Phylogeny and the evolution of host plant use and sequestration in the willow leaf beetle genus Phratora (Coleoptera: Chrysomelidae). Evolution 52:517–528
Kruse JJ, Raffa KF (1999) Effect of food plant switching by a herbivore on its parasitoid: Cotesia melanoscela development in Lymantria dispar exposed to reciprocal dietary crosses. Ecol Entomol 24:37–45
Lawton JH (1986) The effect of parasitoids on phytophagous insect communities. In: Waage J, Greathead D (eds) Insect parasitoids. Academic Press, London, pp 265–289
Lundvall P, Neuvonen S, Halonen M (1998) Interspecific differences in the susceptibility of adult leaf beetles (Coleoptera: Chrysomelidae) to predation by willow warbles (Phylloscopus trochilus). Rep Kevo Subarct Res Stn 22:19–24
Ohsaki N, Sato Y (1990) Avoidance mechanisms of three Pieris butterfly species against the parasitoid wasp Apanteles glomeratus. Ecol Entomol 15:169–176
Oppenheim AJ, Gould F (2002) Behavioral adaptations increase the value of enemy free space for Heliothis subflexa, a specialist herbivore. Evolution 56:679–689
Palokangas P, Neuvonen S (1992) Differences between species and instars of leaf beetles in the probability to be preyed on. Ann Zool Fenn 29:273–278
Paré PW, Lewis WJ, Tumlinson JH (1999) Induced plant volatiles: biochemistry and effects on parasitoids. In: Agrawal AA, Tuzun S, Bent E (eds) Induced plant defenses against pathogens and herbivores. APS, St. Paul, Minn., pp 167–180
Pasteels JM, Gregoire JC, Rowell–Rahier M (1983a) The chemical ecology of defense in arthropods. Annu Rev Entomol 28:263–289
Pasteels JM, Rowell-Rahier M, Braekman JC, Dupont A (1983b) Salicin from host plant as precursor of salicylaldehyde in defensive secretion of chrysomeline larvae. Physiol Entomol 8:307–314
Petitt FL, Wietlisbach DO (1995) Effects of host instar and size on parasitization efficiency and life history parameters of Opius dissitus. Entomol Exp Appl 66:227–236
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Rank NE (1992) Host plant preference based on salicylate chemistry in a willow leaf beetle (Chrysomela aeneicollis). Oecologia 90:95–101
Rank NE (1994) Host plant effects on larval survival in a salicin–using leaf beetle Chrysomela aeneicollis (Coleoptera: Chrysomelidae). Oecologia 97:342–353
Rank NE, Smiley JT (1994) Host-plant effects on Parasyrphus melanderi Curran (Diptera: Syrphidae) feeding on a willow leaf beetle Chrysomela aeneicollis Schaeffer (Coleoptera: Chrysomelidae). Ecol Entomol 19:31–38
Rank NE, Smiley JT, Köpf A (1996) Natural enemies and host plant relationships for chrysomeline leaf beetles feeding on Salicaceae. In: Jolivet PH, Cox ML (eds) Chrysomelidae biology, vol 2: Ecological studies. SPB, Amsterdam, pp 147–171
Rank NE, Köpf A, Julkunen-Tiitto R, Tahvanainen J (1998) Host preference and larval performance of the salicylate-using leaf beetle Phratora vitellinae. Ecology 79:618–631
Richter VA, Zvereva EL (1996) The tachinid species Cleonice nitidiuscula Zetterstedt new for fauna of Murmansk Province (Diptera: Tachinidae). Zoosyst Ross 6:202
Rowell-Rahier M, Pasteels JM (1982) The significance of salicin for a Salix-feeder, Phratora (Phyllodecta) vitellinae. In: Visser JH, Minks AK (eds) Proceedings of the 5th International Symposium on Insect-Plant Relationships. Pudoc, Wageningen, pp 73–79
Salt G (1963) The defense reactions of insects to metazoan parasites. Parasitology 53:527–642
Schulz S, Gross J, Hilker M (1997) Origin of defensive secretion of the leaf beetle Chrysomela lapponica. Tetrahedron 53:9203–9212
Sears ALW, Smiley JT, Hilker M, Muller F, Rank NE (2001) Nesting behavior and prey use in two geographically separated populations of the specialist wasp Symmorphus cristatus (Vespidae: Eumeninae). Am Midl Nat 145:233–246
Sequeira R, Mackauer M (1992) Nutritional ecology of an insect host–parasitoid association: the pea-aphid-Aphidius ervi system. Ecology 73:183–189
Smiley JT, Horn JH, Rank NE (1985) Ecological effects of salicin at three trophic levels: new problems from old adaptations. Science 229:649–651
Stamp N (2001) Enemy-free space via host plant chemistry and dispersion: assessing the influence of tri-trophic interactions. Oecologia 128:153–163
Tahvanainen J, Julkunen-Tiitto R, Kettunen J (1985b) Phenolic glycosides govern the food selection pattern of willow feeding leaf beetles. Oecologia 67:52–56
Termonia A, Hsiao TH, Pasteels JM, Milinkovitch MC (2001) Feeding specialization and host-derived chemical defense in Chrysomeline leaf beetles did not lead to an evolutionary dead end. Proc Natl Acad Sci USA 98:3909–3914
Thompson JN (1988a) Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol Exp Appl 47:3–14
Thompson JN (1988b) Evolutionary genetics of oviposition preference in swallowtail butterflies. Evolution 42:1223–1234
Topp W, Beracz P, Zimmerman K (1989) Distribution pattern, fecundity, development, and survival of Melasoma vigintipunctata (Scop.) (Coleoptera: Chrysomelidae). Entomography 6:355–371
Turlings TCJ, Benrey B (1998) Effects of plant metabolites on behavior and development of parasitic wasps. Ecoscience 5:1–13
Turlings TCJ, Tumlinson JH, Lewis WJ, Vet LEM (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253
Van den Bosch R (1964) Encapsulation of eggs of Bathyplectes curculionis (Thompson) (Hymenoptera: Ichneumonidae) in larvae of Hypera brunneipennis (Boheman) and Hypera postica (Gyllenhal) (Coleoptera, Curculionidae). J Insect Pathol 6:343–367
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Wallace JB, Blum MS (1969) Refined defensive mechanisms in Chrysomela scripta. Ann Entomol Soc Am 62:503–506
Wen B, Weaver DK, Brower JH (1995) Size preference and sex ration for Pteromalus cerealellae (Hymenoptera: Pteromalidae). Environ Entomol 24:1160–1166
Williams IS, Jones TH, Hartley SE (2001) The role of resources and natural enemies in determining the distribution of an insect herbivore population. Ecol Entomol 26:204–211
Yamaga Y, Ohgushi T (1999) Preference–performance linkage in a herbivorous lady beetle: Consequences of variability of natural enemies. Oecologia 119:183–190
Zvereva EL, Kozlov MV (2000) Effects of air pollution on natural enemies of the leaf beetle Melasoma lapponica. J Appl Ecol 37:298–308
Zvereva EL, Kozlov MV, Neuvonen S (1995a) Decrease of feeding niche breadth of Melasoma lapponica (Coleoptera: Chrysomelidae) with increase of pollution. Oecologia 104:323–329
Zvereva EL, Kozlov MV, Neuvonen S (1995b) Population density and performance of Melasoma lapponica (Coleoptera: Chrysomelidae) in surroundings of a smelter complex. Environ Entomol 24:707–715
Zvereva EL, Kozlov MV, Haukioja E (1997) Population dynamics of a herbivore in an industrially modified landscape: case study with Melasoma lapponica (Coleoptera: Chrysomelidae). Acta Phytopathol Entomol Hung 32:251–258
Acknowledgements
We thank V. Zverev for assistance in the fieldwork and R. H. L. Disney and V. Richter for the discussions on parasitoid biology. We are very grateful to E. Dahlhoff, M. Kozlov, S. Neuvonen, H. Roininen, J. Tahvanainen and three anonymous reviewers for valuable comments on earlier drafts of the manuscripts. This study was financially supported by the Maj and Tor Nessling Foundation and the Turku University Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zvereva, E.L., Rank, N.E. Host plant effects on parasitoid attack on the leaf beetle Chrysomela lapponica . Oecologia 135, 258–267 (2003). https://doi.org/10.1007/s00442-003-1184-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1184-9