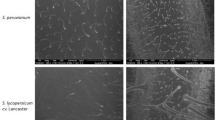
Abstract
Many hypotheses have been proposed to explain the adaptive significance of aggregative feeding in the Lepidoptera. One hypothesis that has received little attention is how induced plant responses may be influenced by aggregative feeding, as compared to feeding by solitary larvae. This study investigated the role of aggregative feeding of the pipevine swallowtail, Battus philenor, in California with special emphasis on the induced responses to herbivory of its hostplant. Here, I show that first-instar larvae develop faster when feeding in a large aggregation compared to solitary or small groups of larvae. Furthermore, I show that this effect is mediated by a larval-density-dependent response in the plant and is independent of prior larval experience and direct interaction among larvae. These results indicate that large groups of larvae can effectively enhance hostplant suitability. A separate experiment showed that larvae feeding on previously damaged leaves had a reduced growth rate. Thus, following initial damage a plant first goes through a period of increased suitability, followed by induced resistance against subsequent herbivory. Aggregative feeding in this system may be an adaptive strategy for larvae to manipulate hostplant suitability, adding a new dimension to the role of aggregative feeding for the Lepidoptera.



Similar content being viewed by others
References
Agrawal AA (1998) Induced responses to herbivory and increased plant performance. Science 279:1201–1202
Agrawal AA (1999) Induced responses to herbivory in wild radish: Effects on several herbivores and plant fitness. Ecology 80:1713–1723
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Axén AH, Pierce NE (1998) Aggregation as a cost-reducing strategy for lycaenid larvae. Behav Ecol 9:109–115
Baldwin IT (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA 95:8113–8118
Baldwin IT, Ohnmeiss TE (1994) Coordination of photosynthetic and alkaloidal responses to damage in uninducible and inducible Nicotiana sylvestris. Ecology 75:1003–1014
Benrey B, Denno RF (1997) The slow-growth-high-mortality hypothesis: A test using the cabbage butterfly. Ecology 78:987–999
Berryman AA, Dennis B, Raffa KF, Stenseth NC (1985) Evolution of optimal group attack, with particular reference to bark beetles (Coleoptera: Scolytidae). Ecology 66:898–903
Broadway RM (1996) Dietary proteinase inhibitors alter complement of midgut proteases. Arch Insect Biochem Physiol 32:39–53
Broadway RM (1997) Dietary regulation of serine proteinases that are resistant to serine proteinase inhibitors. J Insect Physiol 43:855–874
Brower JVZ (1958) Experimental studies of mimicry in some North American butterflies. Part II. Battus philenor and Papilio troilus, P. polyxenes and P. glaucus. Evolution 12:123–136
Bryant SR, Thomas CD, Bale JS (2000) Thermal ecology of gregarious and solitary nettle-feeding nymphalid butterfly larvae. Oecologia 122:1–10
Cahill JF, Castelli JP, Casper BB (2001) The herbivory uncertainty principle: visiting plants can alter herbivory. Ecology 82:307–312
Campo M del , Renwick JAA (2000) Induction of host specificity in larvae of Manduca sexta: chemical dependence controlling host recognition and developmental rate. Chemoecology 10:115–121
Chew FS, Robbins RK (1984) Egg-laying in butterflies. In: Vane-Wright RI, Ackery PR (eds) The biology of butterflies. Academic Press, London, pp 65–79
Clancy KM, Price PW (1987) Rapid herbivore growth enhances enemy attack: sublethal plant defenses remain a paradox. Ecology 68:733–737
Clark BR, Faeth SH (1997) The consequences of larval aggregation in the butterfly Chlosyne lacinia. Ecol Entomol 22:408–415
Cohen MB, Berenbaum MR, Schuler MA (1989) Induction of cytochrome P450-mediated detoxification of xanthotoxin in the black swallowtail. J Chem Ecol 15:2347–2356
Cook A, Denno RF (1994) Planthopper/plant interactions: feeding behavior, plant nutrition, plant defense and host plant specialization. In: Denno RF, Perfect TJ (eds) Planthoppers: their ecology and management. Chapman and Hall, New York, pp 114–139
Courtney SP (1982) Coevolution of pierid butterflies and their cruciferous foodplants. V. Habitat selection, community structure and speciation. Oecologia 54:101–107
Courtney SP (1984) The evolution of egg clustering by butterflies and other insects. Am Nat 123:276–281
Damman H (1991) Oviposition behavior and clutch size in a group-feeding pyralid moth, Omphalocera munroei. J Anim Ecol 60:193–204
De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
Denno RF, Benrey B (1997) Aggregation facilitates larval growth in the neotropical nymphalid butterfly Chlosyne janais. Ecol Entomol 22:133–141
Dicke M, Van Baarlen P, Wessels R, Dijkman H (1993) Herbivory induces systemic production of plant volatiles that attract predators of the herbivore: Extraction of endogenous elicitor. J Chem Ecol 19:581–599
Dixon AFG, Wratten SD (1971) Laboratory studies on aggregation, size and fecundity in the black bean aphid, Aphis fabae Scop. Bull Entomol Res 61:97–111
Dussourd DE (1997) Plant exudates trigger leaf-trenching by cabbage loopers, Trichoplusia ni (Noctuidae). Oecologia 112:362–369
Dussourd DE, Eisner T (1987) Vein-cutting behavior insect counterploy to the latex defense of plants. Science 237:898–901
Fisher RA (1930) The genetical theory of natural selection. Oxford University Press, Oxford
Fitzgerald TD (1993) Sociality in caterpillars. In: Stamp NE, Casey TM (eds) Caterpillars: ecological and evolutionary constraints on foraging. Chapman and Hall, New York, pp 372–403
Fordyce JA (2000) A model without a mimic: Aristolochic acids from the California pipevine swallowtail, Battus philenor hirsuta, and its host plant, Aristolochia californica. J Chem Ecol 26:2567–2578
Fordyce JA (2001) The lethal plant defense paradox remains: inducible host-plant aristolochic acids and the growth and defense of the pipevine swallowtail. Entomol Exp Appl 100:339-346
Fordyce JA, Agrawal AA (2001) The role of plant trichomes and caterpillar group size on growth and defence of the pipevine swallowtail Battus philenor. J Anim Ecol 70:997–1005
Fordyce JA, Shapiro AM (2003) Another perspective on the slow growth / high mortality hypothesis: chilling effects on swallowtail larvae. Ecology (in press)
Gagliardo A, Guilford T (1993) Why do warning-coloured prey live gregariously? Proc R Soc Lond B Biol Sci 251:69–74
Ghent AW (1960) A study of the group-feeding behaviour of larvae of the jack pine sawfly, Neodiprion pratti banksianae Roh. Behaviour 16:110–148
Havill NP, Raffa KF (2000) Compound effects of induced plant responses on insect herbivores and parasitoids: Implications for tritrophic interactions. Ecol Entomol 25:171–179
Hunter AF (2000) Gregariousness and repellent defences in the survival of phytophagous insects. Oikos 91:213–224
Hunter MD, Schultz JC (1993) Induced plant defenses breached? Phytochemical induction protects an herbivore from disease. Oecologia 94:195–203
Jongsma MA, Bolter C (1997) The adaptation of insects to plant protease inhibitors. J Insect Physiol 43:885–895
Jongsma MA, Bakker PL, Peters J, Bosch D, Stiekema WJ (1995) Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc Natl Acad Sci USA 92:8041–8045
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Karowe DN (1989) Facultative monophagy as a consequence of prior feeding experience: behavioral and physiological specialization in Colias philodice larvae. Oecologia 78:106–111
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144
Lawrence WS (1990) The effects of group size and host species on development and survivorship of a gregarious caterpillar Halisidota caryae (Lepidoptera: Arctiidae). Ecol Entomol 15:53–62
Leather SR, Walsh PJ (1993) Sub-lethal plant defences: the paradox remains. Oecologia 93:153–155
Lee K, Berenbaum MR (1990) Defense of parsnip webworm against phototoxic furanocoumarins: Role of antioxidant enzymes. J Chem Ecol 16:2451–2460
Le Masurier AD (1994) Costs and benefits of egg clustering in Pieris brassicae. J Anim Ecol 63:677-685
Li X, Schuler MA, Berenbaum MR (2002) Jasmonate and salicylate induce expression of herbivore cytochrome P450 genes. Nature 419:712–715
Peterson CH, Black R (1994) An experimentalist's challenge: when artifacts of intervention interact with treatments. Mar Ecol Prog Ser 111:289–297
Pilson D, Rausher MD (1988) Clutch size adjustment by a swallowtail butterfly. Nature 333:361–363
Porter K (1982) Basking behaviour in larvae of the butterfly Euphydryas aurinia. Oikos 38:308–312
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Racheli T, Pariset L (1992) II genere Battus tassonomia e storia naturale. Fragm Entomol [Suppl] 23:1–163
Raffa KF, Berryman AA (1983) The role of host plant resistance in the colonization behavior and ecology of bark beetles (Coleoptera: Scolytidae). Ecol Monogr 53:27–49
Rathcke BJ, Poole RW (1975) Coevolutionary race continues: butterfly larval adaptation to plant trichomes. Science 187:175–176
Rausher MD (1995) Behavioral ecology of oviposition in the pipevine swallowtail, Battus philenor. In: Scriber JM, Tsubaki Y, Lederhouse RC (eds) Swallowtail butterflies: their ecology and evolutionary biology. Scientific Publishers, Gainesville, pp 53–62
Rhoades DF (1985) Offensive-defensive interactions between herbivores and plants: their relevance in herbivore population dynamics and ecological theory. Am Nat 125:205–238
Rothschild M, Reichstein T, von Euw J, Aplin R, Harman RRM (1970) Toxic Lepidoptera. Toxicon 8:293–299
Schultz JC (1983) Habitat selection and foraging tactics of caterpillars in heterogeneous trees. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 61–90
Shapiro AM (1981) The pierid red-egg syndrome. Am Nat 117:276–294
Sillén-Tullberg B (1988) Evolution of gregariousness in aposematic butterfly larvae: A phylogenetic analysis. Evolution 42:293–305
Sillén-Tullberg B, Leimar O (1988) The evolution of gregariousness in distasteful insects as a defense against predators. Am Nat 132:723–734
Sime K (2002) Chemical defence of Battus philenor larvae against attack by the parasitoid Trogus pennator. Ecol Entomol 27:337–345
Snyder MJ, Hsu EL, Feyereisen R (1993) Induction of cytochrome P-450 activities by nicotine in the tobacco hornworm, Manduca sexta. J Chem Ecol 19:2903–2916
Spade P, Tyler H, Brown JW (1988) The biology of seven troidine swallowtail butterflies (Papilionidae) in Colima, Mexico. J Res Lepid 26:13–26
Stamp NE (1980) Egg deposition patterns in butterflies: Why do some species cluster their eggs rather than deposit them singly? Am Nat 115:367–380
Stamp NE (1986) Physical constraints of defense and response to invertebrate predators by pipevine caterpillars (Battus philenor: Papilionidae). J Lepid Soc 40:191–205
Tatar M (1991) Clutch size in the swallowtail butterfly, Battus philenor: The role of host quality and egg load within and among seasonal flights in California (USA). Behav Ecol Sociobiol 28:337–344
Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399:686–688
Tullberg BS, Leimar O, Gamberale-Stille G (2000) Did aggregation favour the initial evolution of warning coloration? A novel world revisited. Anim Behav 59:281–287
Turlings TCJ, Loughrin JH, McCall PJ, Rose USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92:4169–4174
Underwood NC (1998) The timing of induced resistance and induced susceptibility in the soybean-Mexican bean beetle system. Oecologia 114:376–381
Wilson EO (1980) Sociobiology: the abridged edition. The Belknap Press of Harvard University Press, Cambridge, Mass.
Young AM, Moffett MW (1979) Studies on the population biology of the tropical butterfly Mechanitis isthmia in Costa Rica. Am Midl Nat 101:309–319
Acknowledgements
This manuscript was improved by comments from Art Shapiro, Rick Karban, Anurag Agrawal, Chris Nice, Rich Van Buskirk, Kenneth Raffa, William Morris, Carol Boggs, and anonymous reviewers. Thanks to Sharon Strauss for use of her micro-balance. Thanks to Dan Tolson and Shorty Boucher of the University of California Natural Reserve System for facilitating work at Stebbins Cold Canyon Ecological Reserve. This study was supported by a Mildred E. Mathias Graduate Student Research Grant (University of California Natural Reserve System), Public Service Research Program and the Putah-Cache Bioregion Project (UC Davis), Jastro-Shields Awards Program (UC Davis), Center for Population Biology (UC Davis), and Sigma-Xi Grants-in-Aid of Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fordyce, J.A. Aggregative feeding of pipevine swallowtail larvae enhances hostplant suitability. Oecologia 135, 250–257 (2003). https://doi.org/10.1007/s00442-003-1177-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1177-8