Abstract
Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) have therapeutic potential in various diseases due to their capacity to transfer bioactive cargoes such as microRNAs (miRNAs or miRs) to recipient cells. The present study isolated EVs from rat MSCs and aimed to delineate their functions and molecular mechanisms in early brain injury following subarachnoid hemorrhage (SAH). We initially determined the expression of miR-18a-5p and ENC1 in hypoxia/reoxygenation (H/R)-induced brain cortical neurons and rat models of SAH induced by the endovascular perforation method. Accordingly, increased ENC1 and decreased miR-18a-5p were detected in H/R-induced brain cortical neurons and SAH rats. After MSC-EVs were co-cultured with cortical neurons, the effects of miR-18a-5p on neuron damage, inflammatory response, endoplasmic reticulum (ER) stress, and oxidative stress markers were evaluated based on ectopic expression and depletion experiments. miR-18a-5p overexpression in brain cortical neurons co-cultured with MSC-EVs was shown to impede neuron apoptosis, ER stress and oxidative stress while augmenting neuron viability. Mechanistically, miR-18a-5p bound to the 3’UTR of ENC1 and reduced its expression, weakening the interaction between ENC1 and p62. Through this mechanism, transfer of miR-18a-5p by MSC-EVs contributed to the eventual inhibition of early brain injury and neurological impairment following SAH. Overall, miR-18a-5p/ENC1/p62 may be a possible mechanism underlying the cerebral protective effects of MSC-EVs against early brain injury following SAH.







Similar content being viewed by others
References
Ahn SH, Savarraj JP, Pervez M, Jones W, Park J, Jeon SB, Kwon SU, Chang TR, Lee K, Kim DH, Day AL, Choi HA (2018) The subarachnoid hemorrhage early brain edema score predicts delayed cerebral ischemia and clinical outcomes. Neurosurgery 83:137–145
Akacha A, Badraoui R, Rebai T, Zourgui L (2020) Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.18561871-11
Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ (2020) Regulatory mechanism of microRNA expression in cancer. Int J Mol Sci 21
Chen C, Yu Q, Xu K, Cai L, Felicia BM, Wang L, Zhang A, Dai Q, Geng W, Wang J, Mo Y (2020a) Electroacupuncture pretreatment prevents ischemic stroke and inhibits Wnt signaling-mediated autophagy through the regulation of GSK-3beta phosphorylation. Brain Res Bull 158:90–98
Chen H, Shi Z, Xing Y, Li X, Fu F (2020b) Fangchinoline attenuates cardiac dysfunction in rats with endotoxemia via the inhibition of ERK1/2 and NF-kappaB p65 phosphorylation. Ann Transl Med 8:1167
Chen Z, Zhang J, Chen Q, Guo J, Zhu G, Feng H (2014) Neuroprotective effects of edaravone after intraventricular hemorrhage in rats. NeuroReport 25:635–640
Danaii S, Shiri S, Dolati S, Ahmadi M, Ghahremani-Nasab L, Amiri A, Kamrani A, Samadi Kafil H, Chakari-Khiavi F, Hojjat-Farsangi M, Malek Mahdavi A, Mehdizadeh A, Yousefi M (2020) The association between inflammatory cytokines and miRNAs with slow coronary flow phenomenon. Iran J Allergy Asthma Immunol 19:56–64
Dhanoa BS, Cogliati T, Satish AG, Bruford EA, Friedman JS (2013) Update on the Kelch-like (KLHL) gene family. Hum Genomics 7:13
Dong JY, Xia KJ, Liang W, Liu LL, Yang F, Fang XS, Xiong YJ, Wang L, Zhou ZJ, Li CY, Zhang WD, Wang JY, Chen DP (2020) Ginsenoside Rb1 alleviates colitis in mice via activation of endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 signaling pathway. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-020-00561-9
Duan S, Wang F, Cao J, Wang C (2020) Exosomes derived from microRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Devel Ther 14:3143–3158
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: Statistical validation. Stroke 26:627–634; discussion 635
Helbok R, Schiefecker AJ, Beer R, Dietmann A, Antunes AP, Sohm F, Fischer M, Hackl WO, Rhomberg P, Lackner P, Pfausler B, Thome C, Humpel C, Schmutzhard E (2015) Early brain injury after aneurysmal subarachnoid hemorrhage: a multimodal neuromonitoring study. Crit Care 19:75
Kim M, Chin YW, Lee EJ (2017) α, γ-Mangostins induce autophagy and show synergistic effect with gemcitabine in pancreatic cancer cell lines. Biomol Ther (Seoul) 25:609–617
Lee H, Ahn HH, Lee W, Oh Y, Choi H, Shim SM, Shin J, Jung YK (2016) ENC1 modulates the aggregation and neurotoxicity of mutant huntingtin through p62 under ER stress. Mol Neurobiol 53:6620–6634
Lei H, Li J, Zhao Z, Liu L (2016) Inhibition of ectodermal-neural cortex 1 protects neural cells from apoptosis induced by hypoxia and hypoglycemia. J Mol Neurosci 59:126–134
Liu FY, Cai J, Wang C, Ruan W, Guan GP, Pan HZ, Li JR, Qian C, Chen JS, Wang L, Chen G (2018) Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NF-kappaB signaling pathway. J Neuroinflammation 15:347
Lushchak VI (2011) Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp Biochem Physiol C Toxicol Pharmacol 153:175–190
Pan B, Liu Y (2015) Effects of duloxetine on microRNA expression profile in frontal lobe and hippocampus in a mouse model of depression. Int J Clin Exp Pathol 8:15454–15461
Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol Ther 23:812–823
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Rass V, Helbok R (2019) Early brain injury after poor-grade subarachnoid hemorrhage. Curr Neurol Neurosci Rep 19:78
Smajilagic A, Aljicevic M, Redzic A, Filipovic S, Lagumdzija A (2013) Rat bone marrow stem cells isolation and culture as a bone formative experimental system. Bosn J Basic Med Sci 13:27–30
Sorensen SS, Nygaard AB, Nielsen MY, Jensen K, Christensen T (2014) miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res 5:711–718
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167:327–334
Supriya M, Christopher R, Indira Devi B, Bhat DI, Shukla D (2020) Circulating microRNAs as potential molecular biomarkers for intracranial aneurysmal rupture. Mol Diagn Ther 24:351–364
Taylor SM, Giuffre E, Moseley P, Hitchcock PF (2019) The microRNA, miR-18a, regulates neuroD and photoreceptor differentiation in the retina of zebrafish. Dev Neurobiol 79:202–219
Tian J, Xu H, Chen G, Wang H, Bi Y, Gao H, Luo Y (2017) Roles of lncRNA UCA1-miR-18a-SOX6 axis in preventing hypoxia injury following cerebral ischemia. Int J Clin Exp Pathol 10:8187–8198
Xiong L, Sun L, Zhang Y, Peng J, Yan J, Liu X (2020) Exosomes from bone marrow mesenchymal stem cells can alleviate early brain injury after subarachnoid hemorrhage through miRNA129-5p-HMGB1 pathway. Stem Cells Dev 29:212–221
Xu W, Li T, Gao L, Zheng J, Yan J, Zhang J, Shao A (2019) Apelin-13/APJ system attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid hemorrhage in rats. J Neuroinflammation 16:247
Yan J, Manaenko A, Chen S, Klebe D, Ma Q, Caner B, Fujii M, Zhou C, Zhang JH (2013) Role of SCH79797 in maintaining vascular integrity in rat model of subarachnoid hemorrhage. Stroke 44:1410–1417
Zhang C, Wang J, Ma X, Wang W, Zhao B, Chen Y, Chen C, Bihl JC (2018a) ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J Cell Mol Med 22:1873–1882
Zhang L, Luo X, Chen F, Yuan W, Xiao X, Zhang X, Dong Y, Zhang Y, Liu Y (2018b) LncRNA SNHG1 regulates cerebrovascular pathologies as a competing endogenous RNA through HIF-1alpha/VEGF signaling in ischemic stroke. J Cell Biochem 119:5460–5472
Zhang W, Xia X, Wang J, Zhu L, Wang J, Wang G, Chen Y, Kim YM (2021) Oxidative stress and genotoxicity of nitenpyram to earthworms (Eisenia foetida). Chemosphere 264:128493
Funding
This work was funded by the Key Project of Health Commission of Sichuan Province (18PJ030,20ZD018), Research Project of Sichuan Medical Association (S21055), Innovation foundation of The Affiliated Hospital of Chengdu University (CDFYCX202209), Foundation of Affiliated Hospital of Chengdu University (YZD2017004 and Y2021008).
Author information
Authors and Affiliations
Contributions
Yamei Zhang and Junying Liu designed the study. Yamei Zhang, Yan Zhou and Zhonglan Zou collated the data, carried out data analyses and produced the initial draft of the manuscript. Junying Liu, Chenchen Xie and Li Ma contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The current study was approved by the Animal Ethic Committee of Affiliated Hospital of Chengdu University and carried out according to the Guide for the Care and Use of Laboratory animals published by the US National Institutes of Health.
Informed consent
Not Applicable.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
441_2023_3754_MOESM1_ESM.docx
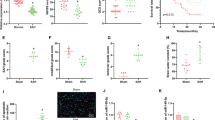
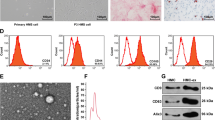
Supplementary Figure 1 Identification of the H/R-induced cell model establishment. a-a’’’, Morphology of primary cortical neurons. b-b’, NSE immunofluorescence staining of primary cortical neurons. c, Neuron viability upon H/R treatment measured by CCK-8 assay. d, Apoptosis rate upon H/R treatment measured by flow cytometry. e, Expression of TNF-α, IL-1β and IL-6 in the supernatant of H/R-treated brain cortical neurons measured by ELISA. f, Western blot of CHOP, GRP78, TXNIP and NLRP3 proteins in H/R-treated brain cortical neurons. g, ROS production in the supernatant of H/R-treated brain cortical neurons measured by ELISA. h, MDA production in the supernatant of H/R-treated brain cortical neurons measured by ELISA. * p < 0.05. Cell experiments were repeated independently three times (n = 3) (DOCX 638 KB)
441_2023_3754_MOESM2_ESM.docx
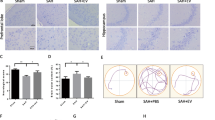
Supplementary Figure 2 Identification of the SAH model establishment. a, Mortality of sham-operated and SAH rats. b, SAH grade of sham-operated and SAH rats. c-d, Neurological function in sham-operated and SAH rats assessed by Modified Garcia Score (C) and beam balance test (D). e, Permeability of BBB in sham-operated and SAH rats assessed by Evans blue staining. f, Brain water content of sham-operated and SAH rats. g, Apoptosis rate of neurons in the brain tissues of sham-operated and SAH rats measured by TUNEL assay. h, Neurodegeneration in the brain tissue of sham-operated and SAH rats shown by FJC staining. i, Expression of TNF-α, IL-1β and IL-6 in the brain tissues of SAH rats measured by ELISA. j, Western blot of CHOP, GRP78, TXNIP and NLRP3 proteins in the brain tissue of SAH rats. k-k’, ROS and MDA production in the brain tissue of SAH rats measured by ELISA. n = 6-8 rats in each group. * p < 0.05 (DOCX 298 KB)
441_2023_3754_MOESM3_ESM.docx
Supplementary Figure 3 Assessment of miR-18a-5p binding to IRF2 and PTGFRN. a-a’, The putaitve binding sites of miR-18a-5p to IRF2 and PTGFRN predicted using TargetScan analysis. b, Binding of miR-18a-5p to IRF2 examined by dual-luciferase reporter assay. c, Binding of miR-18a-5p to PTGFRN examined by dual-luciferase reporter assay. * p < 0.05, compared to the HEK-293T cells transfected with mimic-NC. Cell experiments were repeated independently three times (n = 3) (DOCX 296 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Liu, J., Zhou, Y. et al. miR-18a-5p shuttled by mesenchymal stem cell-derived extracellular vesicles alleviates early brain injury following subarachnoid hemorrhage through blockade of the ENC1/p62 axis. Cell Tissue Res 392, 671–687 (2023). https://doi.org/10.1007/s00441-023-03754-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03754-w