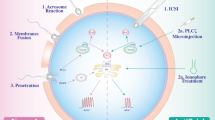
Abstract
Preferentially expressed antigen in melanoma (PRAME) is a cancer/testis antigen (CTA) that is predominantly expressed in normal male gonad tissues and a variety of tumors. PRAME proteins are present in the acrosome and sperm tail, but their role in sperm function is unknown. The objective of this study was to examine the function of the bovine Y-linked PRAME (PRAMEY) during spermatozoal capacitation, the acrosome reaction (AR), and fertilization. Freshly ejaculated spermatozoa were induced to capacitate and undergo AR in vitro. Western blotting results revealed a decrease in the PRAMEY protein in capacitated spermatozoa, and the release of the PRAMEY protein from the acrosome during the AR, suggesting its involvement in sperm capacitation and AR. IVF was performed using in vitro matured bovine oocytes and cauda epididymal spermatozoa either treated with PRAMEY antibody, rabbit IgG, or DPBS. Sperm-egg binding and early embryos were examined at 6 and 45 h post IVF, respectively. The number of spermatozoa that bound per oocyte was nearly two-fold greater in the PRAMEY antibody treatment group (34.4) when compared to both the rabbit IgG (17.6) and DPBS (18.1) controls (P < 0.01). Polyspermy rate in the antibody-treated group (18.9%) was three-fold greater than the rabbit IgG control (6.0%) (P < 0.01). The results indicate that PRAMEY may play a role in anti-polyspermy defense. This study thus provides the initial evidence for the involvement of the PRAME protein family in sperm function and fertilization.






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Abbott A, Ducibella T (2001) Calcium and the control of mammalian cortical granule exocytosis. Front Biosci 6:D792-806
Aitken R, Paterson M, Fisher H, Buckingham D, van Duin M (1995) Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 108:2017–2025
Amann R, Griel LJ (1974) Fertility of bovine spermatozoa from rete testis, cauda epididymidis, and ejaculated sperm. J Dairy Sci 57:212–219
Austin C (1951) Observations on the penetration of the sperm into the mammalian egg. Aust J Sci Res B 4:581
Baldi E, Luconi M, Bonaccorsi L, Krausz C, Forti G (1996) Human sperm activation during capacitation and acrosome reaction: role of calcium, protein phosphorylation and lipid remodelling pathways. Front Biosci 1:189–205
Bergstein-Galan T, Weiss R, Bertol M, Abreu A, Busato E, Kozicki L, Bicudo S (2017) Quality and fertility of frozen ovine spermatozoa from epididymides stored at room temperature (18–25 °C) for up to 48 h post mortem. Theriogenology 96:69–75
Birtle Z, Goodstadt L, Ponting C (2005) Duplication and positive selection among hominin-specific PRAME genes. BMC Genomics 19:1–19
Brucker C, Kaßner G, Löser C, Hinrichsen M, Lipford G (1994) Fertilization and early embryology: progesterone-induced acrosome reaction: potential role for sperm acrosome antigen-1 in fertilization. Hum Reprod 9:1897–1902
Burks D, Carballada R, Moore H, Saling P (1995) Interaction of a tyrosine kinase from human sperm with the zona pellucida at fertilization. Science 269:83–86
Ceulmans H, Bollen M (2004) Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev 84:1–39
Chang M (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168:697
Chang T, Yang Y, Retzel E, Liu W (2013) Male-specific region of the bovine Y chromosome is gene rich with a high transcriptomic activity in testis development. PNAS 110:12373–12378
Chang T, Yang Y, Yasue H, Bharti A, Retzel E, Liu W (2011) The expansion of the PRAME gene family in Eutheria. PLoS ONE 6:e16867
Chen X, Zheng Y, Lei A, Zhang H, Niu H, Li X, Zhang P, Liao M, Lv Y, Zhu Z, Pan C, Dong W, Chen H, Wu D, Liu W, Hamer G, Zeng W (2020) Early cleavage of preimplantation embryos is regulated by tRNAGln-TTG-derived small RNAs present in mature spermatozoa. J Biol Chem 295:10885–10900
Chernoff H, Dukelow W (1969) Decapacitation factor purification with lipid solvents. J Reprod Fertil 18:141–144
Choy M, Moon T, Ravindran R, Bray J, Robinson L, Archuleta T, Shi W, Peti W, Page TK, R, (2019) SDS22 selectively recognizes and traps metal-deficient inactive PP1. PNAS 116:20472–20481
Church D, Goodstadt L, Hillier L, Zody M, Goldstein S, She X, Bult C, Agarwala R, Cherry J, Dicuccio M, Hlavina W, Marques A, Graves T, Zhou S, Teague B, Potamousis K, Churas C, Place M, Herschleb J, Forrest D, Amos-landgraf J, Schwartz D, Lindblad CZ, K, Eichler E, Ponting, C, (2009) Lineage-specific biology revealed by a finished genome assembly of the mouse. Plos Biol 7:e1000112
Cohen P (2002) Protein phosphatase 1—targeted in many directions. J Cell Sci 115:241–256
Costessi A, Mahrour N, Tijchon E, Stunnenberg R, Stoel M, Jansen P, Sela D, Martin-Brown S, Washburn M, Florens L (2011) The tumour antigen PRAME is a subunit of a Cul2 ubiquitin ligase and associates with active NFY promoters. EMBO 30:3786–3798
da Cruz e Silva E, Fox C, Ouimet C, Gustafson E, Watson S, Greengard P, (1995) Differential expression of protein phosphatase 1 isoforms in mammalian brain. J Neurosci 15:3375–3389
Davis B (1978) Inhibition of fertilizing capacity in mammalian spermatozoa by natural and synthetic vesicles. Kabara JJ (ed), Symp Pharmacol Eff Lipids Champaign, Am Oil Chem Soc 145–157
Edwards J, Hansen P (1996) Elevated temperature increases heat shock protein 70 synthesis in bovine two-cell embryos and compromises function of maturing oocytes. Biol Reprod 55:340–346
Epping M, Hart A, Glas A, Krijgsman O, Bernards R (2008) PRAME expression and clinical outcome of breast cancer. Br J Cancer 99:398–403
Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, Bernards R (2005) The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 122:835–847
Fardilha M, Esteves S, Korrodi-Gregorio L, Pelech S, da Cruz ESOA, da Cruz ESA (2011) Protein phosphatase 1 complexes modulate sperm motility and present novel targets for male infertility. Mol Hum Reprod 17:466–477
Huang Z, Khatra B, Bollen M, Carr D, Vijayaraghavan S (2002) Sperm PP1γ2 is regulated by a homologue of the yeast protein phosphatase binding protein sds22. Biol Reprod 67:1936–1942
Igboeli G, Foote R (1968) Maturational changes in bull epididymal spermatozoa. J Dairy Sci Sci 51:1703–1705
Ikeda H, Lethe B, van Baren N, de Smet C, Vitale M, Moretta A, Boon T, Coulie P (1997) Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 6:199–208
Just E (1919) The fertilization reaction in Echinarachinus parma. Biol Bull 36:1–10
Kern C, Feitosa W, Liu W-S (2022) The dynamic of PRAMEY isoforms in testis and epididymis suggests their involvement in spermatozoa maturation. Front Genet 13:846345
Kern C, Yang M, Liu W (2021) The PRAME family of cancer testis antigens is essential for germline development and gametogenesis. Biol Reprod 105:290–304
Kitagawa Y, Sasaki K, Shima H, Shibuya M, Sugimura T, Nagao M (1990) Protein phosphatases possibly involved in rat spermatogenesis. Biochem Biophys Res Commun 171:230–235
Kobe B, Kajava A (2001) The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 11:725–732
Lefebvre R, Chenoweth P, Drost M, LeClear C, MacCubbin M, Dutton J, Suarez S (1995) Characterization of the oviductal sperm reservoir in cattle. Biol Reprod 53:1066–1074
Liu W-S, Zhao Y, Lu C, Ning G, Ma Y, Diaz F, O’Connor M (2017) A novel testis-specific protein, PRAMEY, is involved in spermatogenesis in cattle. Reproduction 153:847–863
Mishra S, Somanath P, Huang Z, Vijayaraghavan S (2003) Binding and inactivation of the germ cell-specific protein phosphatase PP1gamma2 by sds22 during epididymal sperm maturation. Biol Reprod 69:1572–1579
O’Flaherty C, de Lamirande E, Gagnon C (2006) Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med 40:1045–1055
Ojagi M, Kastelic J, Thundathil J (2017) Testis-specific isoform of angiotensin-converting enzyme (tACE) is involved in the regulation of bovine sperm capacitation. Mol Reprod Dev 84:376–388
Oliphant G, Reynold A, Thomas T (1985) Sperm surface components involved in the control of the acrosome reaction. Am J Anat 174:269–284
Parrish J (2014) Bovine In vitro fertilization: In vitro oocyte maturation and sperm capacitation with heparin. Theriogenology 81:67–73
Parrish J, Susko-Parrish J, First N (1989) Capacitation of bovine sperm by heparin: inhibitory effect of glucose and role of intracellular pH. Biol Reprod 41:683–689
Parrish J, Susko-Parrish J, Winer M, First N (1988) Capacitation of bovine sperm by heparin. Biol Reprod 38:1171–1180
Paula-Lopes F, de Moraes AAS, Edwards J, Justice J, Hansen P (1998) Regulation of preimplantation development of bovine embryos by interleukin-1β. Biol Reprod 59:1406–1412
Sellem E, Marthey S, Rau A, Jouneau L, Bonnet A, Le Danvic C, Guyonnet B, Kiefer H, Jammes H, Schibler L (2021) Dynamics of cattle sperm sncRNAs during maturation, from testis to ejaculated sperm. Epigenetic & Chromatin 14:24
Senger PL (2012) Pathways to pregnancy and parturition, 3rd edn. Current Conceptions, Inc.
Shima H, Haneji T, Hatano Y, Kasugai I, Sugimura T, Nagao M (1993a) Protein phosphatase 1 gamma 2 is associated with nuclei of meiotic cells in rat testis. Biochem Biophys Res Commun 194:930–937
Shima H, Hatano Y, Chun Y, Sugimura T, Zhang Z, Lee E, Nagao M (1993b) Identification of PP1 catalytic subunit isotypes PP1 gamma 1, PP1 delta and PP1 alpha in various rat tissues. Biochem Biophys Res Commun 192:1289–1296
Smith G, Wolf D, Trautman K, da Cruz e Silva E, Greengard P, Vijayaraghavan S, (1996) Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol Reprod 54:719–727
Smith G, Wolf D, Trautman K, Vijayaraghavan S (1999) Motility potential of macaque epididymal sperm: the role of protein phosphatase and glycogen synthase kinase-3 activities. J Androl J Androl 20:47–53
Suarez S (1996) Hyperactivated motility in sperm. J Androl 17:331–335
Sutovsky P, Manandhar G, McCauley T, Caamaño J, Sutovsky M, Thompson W, Day B (2004) Proteasomal interference prevents zona pellucida penetration and fertilization in mammals. Biol Reprod 71:1624–1637
Sutovsky P, Ramalho-Santos J, Moreno R, Oko R, Hewitson L, Schatten G (1999) On-stage selection of single round spermatids using a vital, mitochondrion-specific fluorescent probe MitoTracker(TM) and high resolution differential interference contrast microscopy. Hum Reprod 14:2301–2312
Topher-Petersen E, Wagner A, Friedrich J, Petrunkina A, Ekhlasi-Hundrieser M, Waberski D, Drommer W (2002) Function of the mammalian oviductal sperm reservoir. J Exp Zool 292:210–215
Tribulo P, Rivera R, Ortega Obando M, Jannaman E, Hansen P (2019) Production and culture of the bovine embryo. In: Herrick J. (eds) Comparative Embryo Culture. Methods in Molecular Biology. Springer Nature, New York, pp 115–129
Vijayaraghavan S, Stephen D, Trautman K, Smith G, Khatra B, da Cruz e Silva E, Greengard P, (1996) Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol Reprod 54:709–718
Virshup D, Shenolikar S (2009) From promiscuity to precision: protein phosphatases get a makeove. Mol Cell 33:537–545
Visconti P, Bailey J, Moore G, Pan D, Olds-Clarke P, Kopf G (1995) Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 121:1129–1137
Visconti P, Kopf G (1998) Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 59:1–6
Visconti P, Stewart-Savage J, Blasco A, Battaglia L, Miranda P, Kopf G, Tezón J (1999) Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol Reprod 61:76–84
Visconti P, Westbrook V, Chertihin O, Demarco I, Sleight S, Diekman A (2002) Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 53:133–150
Vredenburgh-Wilberg W, Parrish J (1995) Intracellular pH of bovine sperm increases during capacitation. Mol Reprod Dev 40:490–502
Wadelin F, Fulton J, McEwan P, Spriggs K, Emsley J, Heery D (2010) Leucine-rich repeat protein PRAME: expression, potential functions and clinical implications for leukemia. Mol Cancer 9:1–10
Yanagimachi R (1994) Mammalian fertilization. In: Knobil E, Neill J (eds) The Physiology of Reproduction. Raven Press Ltd., New York, pp 189–317
Yue X, Chang T, Dejarnette J, Marshall C, Lei C, Liu W (2013) Copy number variation of PRAMEY across breeds and its association with male fertility in Holstein sires. J Dairy Sci 96:8024–8034
Zeginiadou T, Papadimas J, Mantalenakis S (2000) Acrosome reaction: methods for detection and clinical significance. Andrologia 32:335–343
Zhao Y (2013) Characterization of the PRAME/PRAMEY gene family during spermatogenesis. The Pennsylvania State University.
Acknowledgements
The authors would like to thank Mr. Duane Eichenlaub and Mr. Douglas Nicholas at Nicholas Meat, LLC (Loganton, PA) for providing bovine testes and ovaries. The authors are also grateful to Dr. Bo Harstine at Select Sires, Inc. for providing fresh bovine semen samples.
Funding
This work is supported by the National Institute of Food and Agriculture (NIFA), United States Department of Agriculture (USDA), grant no. 2018–67015-27576 (WSL) and grant no. 2021–67015-33404 (PS).
Author information
Authors and Affiliations
Contributions
WL conceived and designed the research project. CK, WW, CL, JZ, YZ, OMOG, PS, and FD participated in the IVF experimental design. CK, WW, CL, JZ, and YZ carried out IVF experiments. CK, WW, CL, JZ, YZ, OMOG, PS, FD, and WL contributed to the interpretation of the IVF results. CK carried out sperm capacitation and acrosome reaction experiments and data analysis. PS performed PRAMEY/PNA IF staining on spermatids/spermatozoa. CK took the lead in writing the manuscript. WL and PS provided critical feedback and helped shape the research, analysis, and manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kern, C., Wu, W., Lu, C. et al. Role of the bovine PRAMEY protein in sperm function during in vitro fertilization (IVF). Cell Tissue Res 391, 577–594 (2023). https://doi.org/10.1007/s00441-022-03717-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03717-7