Abstract
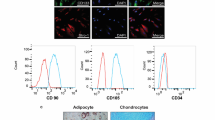
Obesity (Ob) depicts a state of energy imbalance(s) being characterized by the accumulation of excessive fat and which predisposes to several metabolic diseases. Mesenchymal stem cells (MSCs) represent a promising option for addressing obesity and its associated metabolic co-morbidities. The present study aims at assessing the beneficial effects of human placental MSCs (P-MSCs) in mitigating Ob-associated insulin resistance (IR) and mitochondrial dysfunction both in vivo and in vitro. Under obesogenic milieu, adipocytes showed a significant reduction in glucose uptake, and impaired insulin signaling with decreased expression of UCP1 and PGC1α, suggestive of dysregulated non-shivering thermogenesis vis-a-vis mitochondrial biogenesis respectively. Furthermore, obesogenic adipocytes demonstrated impaired mitochondrial respiration and energy homeostasis evidenced by reduced oxygen consumption rate (OCR) and blunted ATP/NAD+/NADP+ production respectively. Interestingly, co-culturing adipocytes with P-MSCs activated PI3K-Akt signaling, improved glucose uptake, diminished ROS production, enhanced mitochondrial OCR, improved ATP/NAD+/NADP+ production, and promoted beiging of adipocytes evidenced by upregulated expression of PRDM16, UCP1, and PGC1α expression. In vivo, P-MSCs administration increased the peripheral blood glucose uptake and clearance, and improved insulin sensitivity and lipid profile with a coordinated increase in the ratio of ATP/ADP and NAD+ and NADP+ in the white adipose tissue (WAT), exemplified in WNIN/GR-Ob obese mutant rats. In line with in vitro findings, there was a significant reduction in adipocyte hypertrophy, increased mitochondrial staining, and thermogenesis. Our findings advocate for a therapeutic application of P-MSCs for improving glucose and energy homeostasis, i.e., probably restoring non-shivering thermogenesis towards obesity management.






Similar content being viewed by others
References
Almeida-Porada G, Atala AJ, Porada CD (2020) Therapeutic mesenchymal stromal cells for immunotherapy and for gene and drug delivery. Mol Ther Methods Clin Dev 16:204–224
Argilés JM, López-Soriano J, Almendro V, Busquets S, López-Soriano FJ (2005) Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med Res Rev 25:49–65
Bernardo ME, Fibbe WE (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13:392–402
Bora P, Majumdar AS (2017) Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8:145
Calderon-Dominguez M, Mir JF, Fucho R, Weber M, Serra D, Herrero L (2016) Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 5:98–118
Cao M, Pan Q, Dong H, Yuan X, Li Y, Sun Z, Dong X, Wang H (2015) Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res Ther 6:208
Chen G, Fan XY, Zheng XP, Jin YL, Liu Y, Liu SC (2020) Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance via PTEN-mediated crosstalk between the PI3K/Akt and Erk/MAPKs signaling pathways in the skeletal muscles of db/db mice. Stem Cell Res Ther 11:401
Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG (2006) Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49:784–791
Chouchani ET, Kazak L, Spiegelman BM (2019) New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab 29:27–37
Cohen P, Spiegelman BM (2016) Cell biology of fat storage. Mol Biol Cell 27:2523–2527
Czech MP (2020) Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol Metab 34:27–42
DeVallance E, Li Y, Jurczak MJ, Cifuentes-Pagano E, Pagano PJ (2019) The role of NADPH oxidases in the etiology of obesity and metabolic syndrome: contribution of individual isoforms and cell biology. Antioxid Redox Signal 31:687–709
Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT (2017) The PI3K pathway in human disease. Cell 170:605–635
Hankir MK, Klingenspor M (2018) Brown adipocyte glucose metabolism: a heated subject. EMBO Rep 19
Harishankar N, Vajreswari A, Giridharan NV (2011) WNIN/GR-Ob - an insulin-resistant obese rat model from inbred WNIN strain. Indian J Med Res 134:320–329
Heinonen S, Muniandy M, Buzkova J, Mardinoglu A, Rodríguez A, Frühbeck G, Hakkarainen A, Lundbom J, Lundbom N, Kaprio J et al (2017) Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: a study of young healthy MZ twins. Diabetologia 60:169–181
Hepler C, Shao M, Xia JY, Ghaben AL, Pearson MJ, Vishvanath L, Sharma AX, Morley TS, Holland WL, Gupta RK (2017). Directing visceral white adipocyte precursors to a thermogenic adipocyte fate improves insulin sensitivity in obese mice. eLife 6
Hruby A, Hu FB (2015) The epidemiology of obesity: a big picture. Pharmacoeconomics 33:673–689
Ikeda K, Maretich P, Kajimura S (2018) The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metab 29:191–200
Ishida M, Tatsumi K, Okumoto K, Kaji H (2020) Adipose tissue-derived stem cell sheet improves glucose metabolism in obese mice. Stem Cells and Development 29:488–497
Jiang H, Yang L, Xing X, Yan M, Guo X, Hou A, Man W, Yang B, Wang Q, Kuang H (2019) A UPLC-MS/MS application for comparisons of the hepatotoxicity of raw and processed Xanthii Fructus by energy metabolites. Rsc Adv 9:2756–2762
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V (2009) Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 5:e1000324
Joffin N, Paschoal VA, Gliniak CM, Crewe C, Elnwasany A, Szweda LI, Zhang Q, Hepler C, Kusminski CM, Gordillo R et al (2021) Mitochondrial metabolism is a key regulator of the fibro-inflammatory and adipogenic stromal subpopulations in white adipose tissue. Cell Stem Cell 28:702–717.e708
Kadam S, Muthyala S, Nair P, Bhonde R (2010) Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud 7:168–182
Kissig M, Ishibashi J, Harms MJ, Lim HW, Stine RR, Won KJ, Seale P (2017) PRDM16 represses the type I interferon response in adipocytes to promote mitochondrial and thermogenic programing. EMBO J 36:1528–1542
Kotikalapudi N, Sampath SJP, Sukesh Narayan SRB, Nemani H, Mungamuri SK, Venkatesan V (2021) The promise(s) of mesenchymal stem cell therapy in averting preclinical diabetes: lessons from in vivo and in vitro model systems. Sci Rep 11:16983
Kotikalapudi N, Sampath SJP, Sukesh Narayan S, Ramesh RB, Nemani H, Mungamuri SK, Venkatesan V (2021b) The promise(s) of mesenchymal stem cell therapy in averting preclinical diabetes: lessons from in vivo and in vitro model systems. Sci Rep 11:16983
Krycer JR, Yugi K, Hirayama A, Fazakerley DJ, Quek LE, Scalzo R, Ohno S, Hodson MP, Ikeda S, Shoji F et al (2017) Dynamic metabolomics reveals that insulin primes the adipocyte for glucose metabolism. Cell Rep 21:3536–3547
Kusminski CM, Scherer PE (2012) Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab 23:435–443
Lecube A, Hernández C, Genescà J, Simó R (2006) Proinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: a case-control study. Diabetes Care 29:1096–1101
Lee YH, Mottillo EP, Granneman JG (2014) Adipose tissue plasticity from WAT to BAT and in between. Biochem Biophys Acta 1842:358–369
Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P et al (2013) Evidence for two types of brown adipose tissue in humans. Nat Med 19:631–634
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370
Liu D, Bordicchia M, Zhang C, Fang H, Wei W, Li JL, Guilherme A, Guntur K, Czech MP, Collins S (2016) Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J Clin Investig 126:1704–1716
Liu Y, Ge X, Dou X, Guo L, Liu Y, Zhou SR, Wei XB, Qian SW, Huang HY, Xu CJ et al (2015) Protein Inhibitor of Activated STAT 1 (PIAS1) Protects against obesity-induced insulin resistance by inhibiting inflammation cascade in adipose tissue. Diabetes 64:4061–4074
Madhira SL, Challa SS, Chalasani M, Nappanveethl G, Bhonde RR, Ajumeera R, Venkatesan V (2012) Promise(s) of mesenchymal stem cells as an in vitro model system to depict pre-diabetic/diabetic milieu in WNIN/GR-Ob mutant rats. PLoS ONE 7:e48061
Maraldi T, Angeloni C, Giannoni E, Sell C (2015) Reactive oxygen species in stem cells. Oxid Med Cell Longev 2015:159080
Mathew SA, Bhonde RR (2018) Omega-3 polyunsaturated fatty acids promote angiogenesis in placenta derived mesenchymal stromal cells. Pharmacol Res 132:90–98
Mathew SA, Chandravanshi B, Bhonde R (2017) Hypoxia primed placental mesenchymal stem cells for wound healing. Life Sci 182:85–92
Mishra VK, Shih HH, Parveen F, Lenzen D, Ito E, Chan TF, Ke LY (2020) Identifying the therapeutic significance of mesenchymal stem cells. Cells 9
Mungamuri SK, Qiao RF, Yao S, Manfredi JJ, Gu W, Aaronson SA (2016) USP7 enforces heterochromatinization of p53 target promoters by protecting SUV39H1 from MDM2-mediated degradation. Cell Rep 14:2528–2537
Mungamuri SK, Wang S, Manfredi JJ, Gu W, Aaronson SA (2015) Ash2L enables P53-dependent apoptosis by favoring stable transcription pre-initiation complex formation on its pro-apoptotic target promoters. Oncogene 34:2461–2470
Mungamuri SK, Yang X, Thor AD, Somasundaram K (2006) Survival signaling by Notch1: mammalian target of rapamycin (mTOR)-dependent inhibition of p53. Can Res 66:4715–4724
Ohno H, Shinoda K, Spiegelman BM, Kajimura S (2012) PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15:395–404
Qi Y, Liu W, Wang X, Lu N, Yang M, Liu W, Ma J, Liu W, Zhang W, Li S (2021) Adipose-derived mesenchymal stem cells from obese mice prevent body weight gain and hyperglycemia. Stem Cell Res Ther 12:277
Rodríguez A, Becerril S, Hernández-Pardos AW, Frühbeck G (2020) Adipose tissue depot differences in adipokines and effects on skeletal and cardiac muscle. Curr Opin Pharmacol 52:1–8
Roman EA, Reis D, Romanatto T, Maimoni D, Ferreira EA, Santos GA, Torsoni AS, Velloso LA, Torsoni MA (2010) Central leptin action improves skeletal muscle AKT, AMPK, and PGC1 alpha activation by hypothalamic PI3K-dependent mechanism. Mol Cell Endocrinol 314:62–69
Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444:847–853
Saltiel AR, Olefsky JM (2017) Inflammatory mechanisms linking obesity and metabolic disease. J Clin Investig 127:1–4
Shang Q, Bai Y, Wang G, Song Q, Guo C, Zhang L, Wang Q (2015) Delivery of adipose-derived stem cells attenuates adipose tissue inflammation and insulin resistance in obese mice through remodeling macrophage phenotypes. Stem Cells Dev 24:2052–2064
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14:493–507
Shree N, Venkategowda S, Venkatranganna MV, Datta I, Bhonde RR (2019) Human adipose tissue mesenchymal stem cells as a novel treatment modality for correcting obesity induced metabolic dysregulation. Int J Obes 2005(43):2107–2118
Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, Shen J, Cheng Y, Fu X, Han W (2012) Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes 61:1616–1625
Singh AM, Zhang L, Avery J, Yin A, Du Y, Wang H, Li Z, Fu H, Yin H, Dalton S (2020) Human beige adipocytes for drug discovery and cell therapy in metabolic diseases. Nat Commun 11:2758
Srivastava RK, Moliner A, Lee ES, Nickles E, Sim E, Liu C, Schwarz H, Ibáñez CF (2020) CD137 negatively affects “browning” of white adipose tissue during cold exposure. J Biol Chem 295:2034–2042
TeSlaa T, Teitell MA (2014) Techniques to monitor glycolysis. Methods Enzymol 542:91–114
Tomas E, Kelly M, Xiang X, Tsao TS, Keller C, Keller P, Luo Z, Lodish H, Saha AK, Unger R et al (2004) Metabolic and hormonal interactions between muscle and adipose tissue. Proc Nutr Soc 63:381–385
Ullah M, Liu DD, Thakor AS (2019) Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience 15:421–438
Vijayalakshmi R, Bamji MS (1987) Altered glucose metabolism in female rats treated with sex steroids: reversal by excess pyridoxine. Indian J Biochem Biophys 24:329–335
Vishvanath L, Gupta RK (2019) Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Investig 129:4022–4031
Wang CH, Lundh M, Fu A, Kriszt R, Huang TL, Lynes MD, Leiria LO, Shamsi F, Darcy J, Greenwood BP et al (2020) CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci Transl Med 12
Wang M, Song L, Strange C, Dong X, Wang H (2018) Therapeutic effects of adipose stem cells from diabetic mice for the treatment of type 2 diabetes. Mol Ther 26:1921–1930
Wang W, Ishibashi J, Trefely S, Shao M, Cowan AJ, Sakers A, Lim HW, O’Connor S, Doan MT, Cohen P et al (2019) A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab 30:174–189.e175
Wang XL, Zhang L, Youker K, Zhang MX, Wang J, LeMaire SA, Coselli JS, Shen YH (2006) Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes 55:2301–2310
Xie Z, Cheng Y, Zhang Q, Hao H, Yin Y, Zang L, Wang X, Mu Y (2021) Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice. Open Life Sci 16:653–666
Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, Sarkar A, Grisotto M, Taneja R, Ghaffari S (2008) Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem 283:25692–25705
Yang C, Wu M, You M, Chen Y, Luo M, Chen Q (2021) The therapeutic applications of mesenchymal stromal cells from human perinatal tissues in autoimmune diseases. Stem Cell Res Ther 12:103
Zachar L, Bačenková D, Rosocha J (2016) Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res 9:231–240
Zhang Z, Huang S, Wu S, Qi J, Li W, Liu S, Cong Y, Chen H, Lu L, Shi S et al (2019) Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine 45:341–350
Acknowledgements
The authors would like to acknowledge Dr. R. Hemalatha, Director, ICMR-NIN, for providing the necessary infrastructure to conduct the current research. We also would like to acknowledge the members of the ICMR-NIN animal facility for their cooperation and support for animal experiments.
Funding
KN received a Senior Research Fellowship (5/3/8/31/ITR-F/2018-ITR) from ICMR. The present research is supported by ICMR-NIN intramural funding (Code: 15-BS03, 18-BS10) to Dr. VV, which is greatly acknowledged.
Author information
Authors and Affiliations
Contributions
KN carried out the majority of experiments, characterized, and injected P-MSCs, including animal maintenance. SJPS also contributed towards animal experiments and towards manuscript. SNS helped in metabolite analysis using LC–MS/MS. RRB is involved in human placenta collection and human ethical approval as well as isolation of P-MSCs. SKM is engaged in designing the experiments, data analysis, the configuration of figures, and preparation and final correction of the manuscript. VV coordinated the overall team, including the project design and manuscript preparation.
Corresponding author
Ethics declarations
Ethics approval
Institutional Animal Ethics Committees (IAECs): P35F/IAEC/NIN/11/2012/VV/WNIN. Institutional Human Ethics Committees: MHB/SCR/021.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kotikalapudi, N., Sampath, S.J.P., Sinha, S.N. et al. Placental mesenchymal stem cells restore glucose and energy homeostasis in obesogenic adipocytes. Cell Tissue Res 391, 127–144 (2023). https://doi.org/10.1007/s00441-022-03693-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03693-y