Abstract
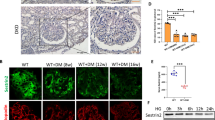
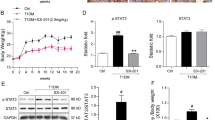
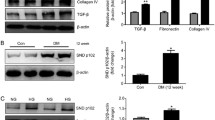
Glomerular mesangial cell proliferation and extracellular matrix accumulation contribute to the progression of diabetic nephropathy (DN). As a conserved stress-inducible protein, sestrin2 (Sesn2) plays critical role in the regulation of oxidative stress, inflammation, autophagy, metabolism, and endoplasmic reticulum stress. In this study, we investigated the role of Sesn2 on renal damage in diabetic kidney using transgenic mice overexpressing Sesn2 and the effect of Sesn2 on mesangial cell proliferation and extracellular matrix accumulation in diabetic conditions and the possible molecular mechanisms involved. Sesn2 overexpression improved renal function and decreased glomerular hypertrophy, albuminuria, mesangial expansion, extracellular matrix accumulation, and TGF-β1 expression, as well as oxidative stress in diabetic mice. In vitro experiments, using human mesangial cells (HMCs), revealed that Sesn2 overexpression inhibited high glucose (HG)-induced proliferation, fibronectin and collagen IV production, and ROS generation. Meanwhile, Sesn2 overexpression restored phosphorylation levels of Lats1 and YAP and inhibited TEAD1 expression. Inhibition of Lats1 accelerated HG-induced proliferation and expression of fibronectin and collagen IV. Verteporfin, an inhibitor of YAP, suppressed HG-induced proliferation and expression of fibronectin and collagen IV. However, Sesn2 overexpression reversed Lats1 deficiency-induced Lats1 and YAP phosphorylation, nuclear expression levels of YAP and TEAD1, and proliferation and fibronectin and collagen IV expressions in HMCs exposed to HG. In addition, antioxidant NAC or tempol treatment promoted phosphorylation of Lats1 and YAP and inhibited TEAD1 expression, proliferation, and fibronectin and collagen IV accumulation in HG-treated HMCs. Taken together, Sesn2 overexpression inhibited mesangial cell proliferation and fibrosis via regulating Hippo pathway in diabetic nephropathy. Induction of Sesn2 may be a potential therapeutic target in diabetic nephropathy.










Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abboud HE (2012) Mesangial cell biology. Exp Cell Res 318(9):979–985
Budanov AV, Karin M (2008) p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134(3):451–460
Budanov AV, Shoshani T, Faerman A et al (2002) Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 21(39):6017–6031
Chen J, Harris RC (2016) Interaction of the EGF receptor and the Hippo pathway in the diabetic kidney. J Am Soc Nephrol 27(6):1689–1700
Chen J, Wang X, He Q et al (2020a) YAP activation in renal proximal tubule cells drives diabetic renal interstitial fibrogenesis. Diabetes 69(11):2446–2457
Chen K, Yu B, Liao J (2021) LncRNA SOX2OT alleviates mesangial cell proliferation and fibrosis in diabetic nephropathy via Akt/mTOR-mediated autophagy. Mol Med 27(1):71
Chen Z, Gao H, Wang L et al (2020b) Farrerol alleviates high glucose-induced renal mesangial cell injury through the ROS/Nox4/ERK1/2 pathway. Chem Biol Interact 316:108921
Cho Y, Park MJ, Kim K et al (2020) Reactive oxygen species-induced activation of Yes-associated protein-1 through the c-Myc pathway is a therapeutic target in hepatocellular carcinoma. World J Gastroenterol 26(42):6599–6613
Collins AJ, Foley RN, Chavers B et al (2014) US renal data system 2013 annual data report. Am J Kidney Dis 63(1 Suppl):A7
Cui W, Min X, Xu X, Du B, Luo P (2017) Role of nuclear factor erythroid 2-related factor 2 in diabetic nephropathy. J Diabetes Res 2017:3797802
Dong J, Feldmann G, Huang J et al (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130(6):1120–1133
Dong Z, Sun Y, Wei G et al (2019) Ergosterol ameliorates diabetic nephropathy by attenuating mesangial cell proliferation and extracellular matrix deposition via the TGF-β1/Smad2 signaling pathway. Nutrients 11(2):483
Eid AA, Lee DY, Roman LJ, Khazim K, Gorin Y (2013) Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol Cell Biol 33(17):3439–3460
Forbes JM, Coughlan MT, Cooper ME (2008) Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57(6):1446–1454
Gorin Y, Block K (2013) Nox4 and diabetic nephropathy: with a friend like this, who needs enemies? Free Radic Biol Med 61:130–142
Halder G, Johnson RL (2011) Hippo signaling: growth control and beyond. Development 138(1):9–22
Ishihara M, Urushido M, Hamada K et al (2013) Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol 305(4):F495-509
Ji F, Shen T, Zou W, Jiao J (2017) UCP2 regulates embryonic neurogenesis via ROS-mediated Yap alternation in the developing neocortex. Stem Cells 35(6):1479–1492
Kai T, Tsukamoto Y, Hijiya N et al (2016) Kidney-specific knockout of Sav1 in the mouse promotes hyperproliferation of renal tubular epithelium through suppression of the Hippo pathway. J Pathol 239(1):97–108
Kanwar YS, Sun L, Xie P, Liu FY, Chen S (2011) A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6:395–423
Kim HJ, Joe Y, Kim SK et al (2017) Carbon monoxide protects against hepatic steatosis in mice by inducing sestrin-2 via the PERK-eIF2alpha-ATF4 pathway. Free Radic Biol Med 110:81–91
Lee JH, Budanov AV, Karin M (2013) Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab 18(6):792–801
Lee JH, Budanov AV, Talukdar S et al (2012) Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab 16(3):311–321
Lee JS, Park HW, Cho KJ et al (2020) Sestrin2 inhibits YAP activation and negatively regulates corneal epithelial cell proliferation. Exp Mol Med 52(6):951–962
Lei D, Chengcheng L, Xuan Q et al (2019) Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway. Pharmacol Res 146:104320
Leung JY, Wilson HL, Voltzke KJ et al (2017) Sav1 loss induces senescence and Stat3 activation coinciding with tubulointerstitial fibrosis. Mol Cell Biol37(12)
Lin CL, Wang JY, Ko JY et al (2010) Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J Am Soc Nephrol 21(1):124–135
Lin Q, Ma Y, Chen Z et al (2020) Sestrin2 regulates podocyte mitochondrial dysfunction and apoptosis under high glucose conditions via AMPK. Int J Mol Med 45(5):1361–1372
Liu Y, Li M, Du X, Huang Z, Quan N (2021) Sestrin 2, a potential star of antioxidant stress in cardiovascular diseases. Free Radic Biol Med 163:56–68
Luo C, Zhao S, Zhang M et al (2018) SESN2 negatively regulates cell proliferation and casein synthesis by inhibition the amino acid-mediated mTORC1 pathway in cow mammary epithelial cells. Sci Rep 8(1):3912
Ma S, Meng Z, Chen R, Guan KL (2019) The Hippo pathway: biology and pathophysiology. Annu Rev Biochem 88:577–604
Pasha M, Eid AH, Eid AA, Gorin Y, Munusamy S (2017) Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid Med Cell Longev 2017:3296294
Qian X, He L, Hao M et al (2021) YAP mediates the interaction between the Hippo and PI3K/Akt pathways in mesangial cell proliferation in diabetic nephropathy. Acta Diabetol 58(1):47–62
Ro SH, Nam M, Jang I et al (2014) Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc Natl Acad Sci U S A 111(21):7849–7854
Schena FP, Gesualdo L (2005) Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol Suppl 1:S30–33
Singh DK, Winocour P, Farrington K (2011) Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol 7(3):176–184
Tinajero MG, Malik VS (2021) An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin North Am 50(3):337–355
Umanath K, Lewis JB (2018) Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 71(6):884–895
Wang S, Wen X, Han XR et al (2018) Repression of microRNA-382 inhibits glomerular mesangial cell proliferation and extracellular matrix accumulation via FoxO1 in mice with diabetic nephropathy. Cell Prolif 51(5):e12462
Wolf G, Ziyadeh FN (1999) Molecular mechanisms of diabetic renal hypertrophy. Kidney Int 56(2):393–405
Young BA, Johnson RJ, Alpers CE et al (1995) Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int 47(3):935–944
Zhang L, Pang S, Deng B et al (2012) High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-kappaB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int J Biochem Cell Biol 44(4):629–638
Zhao B, Ye X, Yu J et al (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22(14):1962–1971
Zhao HJ, Wang S, Cheng H et al (2006) Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17(10):2664–2669
Zheng H, Whitman SA, Wu W et al (2011) Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60(11):3055–3066
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81470966), the Funds for Guiding Local Scientific and Technological Development by the Central Government of China (216Z7703G), and the Natural Science Foundation of Hebei Province (No. H2021206144; H2021206356).
Author information
Authors and Affiliations
Contributions
Yawei Bian, Shan Song, and Yonghong Shi contributed to the conception and design of the study. Yawei Bian, Chonglin Shi, Ming Wu, Jiajia Dong, and Xuan Dong were responsible for the execution of the cell experiments. Shan Song, Duojun Qiu, and Dongyun Wang were responsible for the animal experiments. Lin Mu, Wei Zhang, Zihui Zhou, and Chen Yuan were responsible in the assistance of the completion of animal experiments. Yawei Bian and Yonghong Shi were responsible for writing and editing the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All experimental animal procedures were reviewed and approved by Ethics Review Committee for Animal Experimentation of Hebei Medical University (No. IACUC-Hebmu-2021030). Clinical specimens were approved by Hebei Medical University ethical committees (No. 20190015).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bian, Y., Shi, C., Song, S. et al. Sestrin2 attenuates renal damage by regulating Hippo pathway in diabetic nephropathy. Cell Tissue Res 390, 93–112 (2022). https://doi.org/10.1007/s00441-022-03668-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03668-z